Abstract
Acrylamide (AA) is a potentially carcinogenic substance which is formed during heating of food products containing carbohydrates and asparagine. It was first detected in food products in 2002. Since that time, several analytical methods have been made available for the quantification of AA in various foods. Starting from the announcement in 2002, occurrence, formation, chemistry, toxicology, and potential health risk in the human diet have been investigated and methods of analysis have been reviewed in many articles. In this paper, current information and analytical methods for the determination of AA have been reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food safety and food quality is a matter of public concern and monitored closely by national authorities and the interest of customer preferences for good quality in food products drives the food industry to seek for more efficient, cost-effective, faster, and powerful analytical methods in order to control the quality attributes. Regulations regarding food and trade, describe in detail the requirements of the data to be provided by analytical methods which can be summarized as follows: compliance with quality control specifications, determination of possible contaminations, and chemical composition.
The European Chemicals Agency (ECHA) (2010) added acrylamide (AA) to the list of substances of very high concern. As AA has been classified as a probable carcinogenic substance to humans, national and international regulatory agencies have focused their attention on the detection of AA in food items (US EPA (US Environmental Protection Agency 1994), (European Commission 2001)). It is stated by FDA (US Food and Drug Administration) (2006) that “development of rapid or inexpensive screening methods and validating confirmatory methods of analysis” is a major goal for the issue of AA in food.
AA is a low-molecular-weight vinylic compound. It is a colorless and odorless crystalline substance and it is highly water soluble, easily reactive in air, and rapidly polymerizable. AA is mostly used as a monomer in the production of polyacrylamide which in turn is used in the paper and textile industries, as a flocculant in the treatment of wastewater, as a soil conditioner, in ore processing, and in cosmetics. The monomeric form of acrylamide has been shown to be a potential health hazard for humans and is a known neurotoxic compound (LeQuesne 1980). Moreover, it has been classified as “probably carcinogenic to humans” by the International Agency for Research on Cancer on the basis of sufficient evidence for carcinogenicity in experimental animals and mechanistic considerations (IARC (International Agency for Research on Cancer) 1994).
First attempts for the determination of acrylamide in food products began in 2002 upon a report published by the Swedish National Food Administration, Stockholm (2002), about high levels of AA in heat-treated potato products and other baked goods. Following the report another study confirmed the high levels of AA in fried and baked potato products and in cereal products such as crisp bread, breakfast cereals, and cookies (Svensson et al. 2003).
AA is formed during the heating processes such as frying, roasting, and baking at temperatures around 120 °C reaching its maximum formation peaks at temperatures between 160 °C and 180 °C through interaction of amino acids, especially asparagine, with reducing sugars like glucose (Mottram et al. 2002 and Stadler et al. 2002). AA is not typically found in boiled or microwaved food.
Several analytical methods have been reported for the determination of acrylamide in different types of food since 2002. In this review, the analytical methods for the determination of acrylamide in food products from 2004 to 2010 are presented.
Regulatory Levels in Foods
AA is not formed naturally, but has been found in wide range of food products. Food groups containing higher levels of AA and which are important due to their relatively high consumption rate can be listed as follows; potato products (French fries, oven-baked chips, potato crisps, and so on), different cereal-based foods such as breakfast cereals, cookies, biscuits, bread (especially toasted bread), pies, cakes, coffee, chicory, and other coffee substitutes, chocolate, teething biscuits, baby rusks, and various other baby foods. A joint FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization) consultation group on the health implications of acrylamide in food has estimated an average daily food intake of acrylamide by the general population in the range of 0.3–0.8 μg/kg bw/day (FAO/WHO; Food and Agriculture Organization of the United Nations and World Health Organization 2002). According to recent exposure assessments, the daily dietary intake of AA with diet is approximately 0.4 μg/kg bw with a 90th percentile of 0.95 μg/kg bw (FDA (US Food and Drug Administration) 2006). Permissible levels were established for drinking water by the WHO at 1 μg/L, by the European Union at 1 μg/L, and by the US Environmental Protection Agency (EPA) at 0.5 μg/L.
Formation
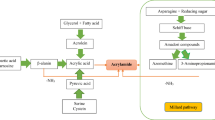
AA is very reactive in foods because of its high solubility in water and reactivity with acids, bases, and oxidizing agents. It is very reactive in foods. As AA is a genotoxic substance, a collaborative effort by national food authorities and the food industry was performed in order to understand its formation in a better way. The formation is reported to occur by the Maillard reaction between asparagine (ASN) and glucose, which might happen during cooking and food processing (Mottram et al. 2002) and is reported to be responsible for the high levels of AA in food that is baked, fried, or roasted and is also responsible for browning and flavor of these foods. There are mechanistic pathways that were proposed to explain AA formation from ASN. Schiff’s base formation occurs after the Maillard reaction between ASN and a carbonyl compound (Mottram et al. 2002; Stadler et al. 2002). According to the most probable pathway assumed, AA is formed by rearrangement after decarboxylation of the Schiff’s base. Another precursor of AA, a biogenic amine 3-aminopropionamide (3-APA) was identified by Granvogl et al. (2004) and Granvogl and Schieberle (2006). During the Maillard reaction 3-APA, appears to be formed (Granvogl and Schieberle 2006; Goldmann et al. 2006). The exact mechanism of AA formation in the Maillard reaction has not yet been fully explained.
Biotransformation
AA is easily absorbed and metabolized by the body. The major metabolic pathways consist of the conjugation of AA to glutathione and its epoxidation to glycidamide (GA) in the liver by cytochrome P450 enzyme. Detoxification of GA could occur by hydrolysis to glyceramide in small amounts, which is mediated by epoxide hydrolase (Sumner et al. 1992). AA does not cause mutagenesis in Salmonella assays in the presence or absence of a microsomal activating system. GA causes, however, mutagenesis in similar systems and is the reactive epoxide metabolite of AA (Hashimoto and Tanii 1985). Sumner et al. (1992) showed that AA is metabolized to GA via cytochrome P450 enzymes. They compared AA metabolism in wild-type mice, mice pretreated with a P450 inhibitor, and mice lacking cytochrome P450 2E1. Conjugation products of GA with glutathione may also occur and are excreted in urine after metabolic conversion to the regioisomeric mercapturic acids rac-N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA) and rac-N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-l-cysteine (iso-GAMA) as shown in the Fig. 1. It is suggested by the comparable induction of micronuclei in rodents given either GA or AA that GA is the dominant genotoxin formed from AA (Paulsson et al. 2003). The most reactive and biologically most important metabolite of AA is GA (Stadler et al. 2002). After exposure to AA in food, GA has been found in human urine (Fennell et al. 2006).
Analytical Methods
Many analytical methods have been reported since the announcement of the AA findings in 2002. In this review, research for the analysis of AA in foods for the years since are covered and presented in details. For the determination of AA, high-performance liquid chromatographic (HPLC), gas chromatographic (GC), and capillary electrophoresis (CE) separations have been used. Of these techniques HPLC has been used most frequently.
Tables 1, 2, and 3 summarize detailed information about the analytical methods (CE, GC, and HPLC) for the analysis of AA in foods. It includes sample pre-treatment; separation and detection techniques; different food samples; and method detection limits.
Capillary Electrophoresis
Microemulsion electrokinetic chromatography was developed for the determination of AA in brownish-colored home-made French fries (Bermudo et al. 2004). A concentration of 6.99 mg/kg was measured with a relative standard deviation of 9.8%. The results showed that the method could be used for quantitative purposes on either food or environmental samples. A nonaqueous CE method was developed for the quantitative determination of AA in processed food like potato chips and French fries (Başkan and Erim 2007). The AA amount of these samples were found to be 2.95 ± 0.11 mg/kg. A field amplified sample stacking technique with the nonaqueous capillary electrophoresis method was introduced for the on-line concentration of AA to increase the poor sensitivity of UV detection at 210 nm by diode array detection (Tezcan and Erim 2008). The application of the sample stacking was performed on potato chips and almond extracts. The AA content in almond extract and some potato chips was found to be 95.5 ± 7.6 and 4.3 ± 1.5 μg/kg, respectively.
Two in-line preconcentration capillary zone electrophoresis (CZE) methods (field amplified sample injection (FASI)) and stacking with sample matrix removal were proposed for the analysis of AA in foodstuffs after derivatization with 2-mercaptobenzoic acid (Bermudo et al. 2006a, b). In order to evaluate the applicability of this FASI–CZE method for the determination of AA in different kinds of food products, eight foodstuffs (including biscuits, crisp bread, cereals, snacks, potatoes, and coffee) were analyzed. The results obtained using both quantification methods were 193 ± 23 and 175 ± 20 μg/kg (biscuits Flora) for standard addition and external calibration, respectively, and 215 ± 21 and 226 ± 18 μg/kg (snacks Doritos). The results were compared to those obtained by liquid chromatography (LC)–mass spectrometry (MS) and similar results were obtained. A CE-MS method was developed for the analysis of AA after its derivatization with 2-mercaptobenzoic acid (Bermudo et al. 2007). For the applicability of the FASI-CE-MS-MS method different kinds of foodstuffs, five representative food products (potato crisps, crisp bread, biscuits, breakfast cereals, and coffee) were analyzed. The results obtained were in agreement with those of liquid chromatography tandem mass spectrometry (LC-MS-MS).
Micellar electrokinetic capillary chromatography was developed for the separation and determination of AA in potato chips at low levels (Zhou et al. 2007). For the applicability of this developed method in real samples, an analysis of potato chips was performed. The obtained values were in agreement with the results reported by FDA, which ranged from 0.693 to 2.510 mg/kg in potato chips.
Gas Chromatography
GC methods have generally involved the derivatization of AA with potassium bromate and potassium bromide in order to improve the GC properties. A recent GC method employing a high-resolution time-of-flight mass analyzer was developed for the direct analysis (no derivatization) of acrylamide in various heat-processed foodstuffs (Dunovska et al. 2006). Extraction with n-propanol followed by solvent exchange MeCN avoided co-isolation of acrylamide precursors that could yield additional analyte in the hot splitless GC injector. Another GC method was based on derivatization of the target analyte with bromination and detection by an electron capture detector (ECD) (Zhang et al. 2006). Their results from the GC-ECD analysis were confirmed with GC-MS. Another GC-MS method to determine AA in coffee and coffee products was developed by Soares et al. (2006). They found AA levels in standard espresso coffee to be 0.32–1.46 μg/30 mL or 10.7–48.7 μg/L.
A later study by these researchers focused exclusively on this particular beverage, ascertaining the factors to be considered in the AA extraction, thus contributing to a better knowledge of the exposure levels for espresso coffee consumers (Alves et al. 2010). Espresso coffees were analyzed for AA by matrix solid-phase dispersion (MSPD) and GC-MS. Mean AA contents of medium-roasted espressos (30 mL) were 1.16 ± 0.25 and 2.31 ± 0.43 μg for pure arabica and robusta samples, respectively. According to another study, with MSPD-GC-MS the AA levels were determined in coffee and coffee substitute samples. The limit of detection (LOD) and limit of quantification (LOQ) values were 5 and 10 μg/kg, respectively (Soares et al. 2010).
A European inter-laboratory study was conducted to validate two analytical procedures (GC-MS) and high-performance liquid chromatography with tandem mass spectrometry for the determination of AA in bakery products (crispbreads, biscuits) and potato products (chips) (Wenzl et al. 2006). The concentration ranges were found to lie between 20 μg/kg and about 9,000 μg/kg. The performance of the LC-MS-MS method was found to be superior to that of the GC-MS method. Development of a MSPD-GC-MS procedure for the analysis of acrylamide in potato chips was presented by Fernandes and Soares (2007). After bromination of the extract the samples were analyzed by GC-MS in a selected ion monitoring mode. Seventeen potato chips samples were simultaneously analyzed by an alternative method based on the extraction of AA with hot water to evaluate the performance of the developed method. The results were found to be very similar using both methods. In another MSPD extraction technique, a modified version of a previous method (Fernandes and Soares 2007) was applied to the extraction of AA from foodstuff such as breakfast cereals, bread, toasted bread, salty snacks, biscuits and cookies, baby food, and chocolate bars, which generally contain low amounts (<100 μg/kg for most of the samples) of acrylamide using GC-MS in the SIM mode (Soares and Fernandes 2009). The MSPD method was found to be more expeditious, simple, and easier to use than liquid extraction methods. The LOD and LOQ values were 5.2 and 15.7 μg/kg, respectively.
Two analytical methods for the determination of AA in Chinese traditional carbohydrate-rich foods such as fried bread sticks, hemp flowers, and Chinese corn crisps were developed by (Zhang et al. 2007a, b). One is based on derivatization with potassium bromate and potassium bromide without clean-up prior to gas chromatography with a micro-electron capture detector. In the second method, the underivatized AA was assayed by high-performance liquid chromatography coupled to quadrupole tandem mass spectrometry in the positive electrospray ionization mode. AA contaminant was found in all of the samples at concentrations up to 771.1 and 734.5 μg/kg detected by the GC and HPLC method, respectively. A GC-ECD method was developed for identification and quantification of AA in heat-processed starchy foods (Zhu et al. 2008). This method has some advantages comparing to MS detection-based methods because the GC-ECD equipments are more cost-effective and generally exists in routine laboratory. A GC-MS-MS method was developed to measure acrylamide in aqueous matrices extracted from French fries and potato crisps by using direct immersion solid-phase microextraction (SPME) without derivatization (Lee et al. 2007). The concentrations of AA detected in French fries and potato crisps were 1.2 and 2.2 μg/g, respectively. Another SPME-GC method was described for the determination of AA in fried foods (Chen et al. 2009). AA was brominated and transformed to 2-bromoacrylamide (2-BAM). 2-BAM was then extracted by a commercial SPME fiber for GC detection. The detection limit of the SPME-GC for 2-BAM was found to be 0.1 μg/L.
High-Performance Liquid Chromatography
Although the CE and GC methods for AA determination are in routine use in some laboratories, the majority of laboratories use HPLC methods. LC, coupled with MS-MS detection is the most preferred. A method using normal-phase high-performance liquid chromatography with UV detection was developed for the analysis of AA and methacrylamide by Paleologos and Kontominas (2005). The method was applied for the determination of AA and methacrylamide in spiked food samples without native AA yielding recoveries between 95% and 103%. A HPLC method with UV detection was developed for the determination of AA in deep-fried flour-based leaven dough foods available in Hong Kong (Wang et al. 2008). The amounts of AA in eight food samples were 27–198 μg/kg when 1 g sample was analyzed.
LC coupled to diode array detection (DAD) was developed for the determination of AA in potato-based foods at low levels (Gökmen et al. 2005). The DAD was set at 226 nm. LC-MS analyses at atmospheric pressure chemical ionization confirmed the results obtained by LC-DAD analyses. An ion-exclusion liquid chromatography coupled with diode array detection (LC-DAD) method was developed for the determination of AA in starch-based foods (Geng et al. 2008). Unlike the poor retention on conventional LC reversed-phase sorbents, AA was separated as a protonated cation and was well resolved from co-extractives. The AA levels in starch-based foods were found to range from 100 to 2,000–3,000 mg/kg. The method might be applicable to a wide range of food categories such as potato chips, French fries, bakery products, and Chinese fried/baked foods.
A LC-MS method was developed for the quantification of AA in coffee, cocoa, and high-salt foods (Eberhart et al. 2005). For difficult food matrixes, such as coffee and cocoa, a solid-phase extraction clean-up step had to be used. The method had a limit of quantification of 10 μg/kg. A LC-MS method based on a stable isotope dilution assay was reported for AA content of commercial potato chips, a major source of AA in the diet (Rufián-Henares and Morales 2006). To obtain high recoveries (98.8%), a critical step consisted of the use of virgin olive oil, as the retaining matrix. Another LC-MS method was reported for the determination of AA in protein-rich foods such as grilled meat and chicken foods as well as carbohydrate-rich foods containing potato chips and biscuit (Kaplan et al. 2009). The obtained concentrations for all studied foods were in the range of 20–250 μg/kg. Large variation in the values in the concentrations of AA even for the same food samples were observed. The result of different processing temperatures, length of heating, cooking type, and different raw materials were responsible for the large variations.
A LC-MS-MS method was developed for analyzing AA in 110 samples of brewed coffee using electrospray in the positive mode (Granby and Fagt 2004). The AA content was found to be 2–16 μg/L in brewed coffee and comparable to FDA results of 6–11 μg/L. Two different sample preparation methods for LC-MS-MS analysis were developed for the analysis of AA over a wide range of different food products (Hoenicke et al. 2004). The first method was applicable to various foods like potato chips, French fries, cereals, bread, and roasted coffee, allowing daily analysis of up to 60 samples per technician. The second preparation method was not as simple and fast but enabled analysis of difficult matrices like cacao, soluble coffee, molasses, and malt. An LC-MS-MS method was used to analyze 112 composite samples prepared from 547 individual carbohydrate-based foods in the Australian diet, including breads, cakes, breakfast cereals, and snack foods (Croft et al. 2004). The method allowed detection at 25 μg/kg.
Ion trap liquid chromatography LC-MS-MS was evaluated for use in the determination of AA in processed foods (Tsutsumiuchi et al. 2004). In analyses of 37 commercial foods, AA was detected in a potato snack at the maximum value of 3.57 ng/g and found in 23 foods prepared or cooked at high temperature. LC-MS-MS interfaced with electrospray was developed for the determination of AA in cooked food samples (Calbiani et al. 2004). It required a sample treatment procedure using an extraction step with acidified water without clean-up.
The AA levels in breast milk and the main categories of Swedish baby food products, breast milk substitute (infant formula), gruel, porridge, and canned baby food, were analyzed using the LC-MS-MS method (Fohgelberg et al. 2005). The results showed great variations in AA levels between and within the different food categories, <0.5–64 μg/kg. Isotope dilution liquid chromatography coupled with electrospray ionization tandem mass spectrometry was applied to the quantification of AA in chocolate matrixes (dark chocolate, milk chocolate, chocolate with nuts, chocolate with almonds, and chocolate with wheat germ) (Ren et al. 2006). The AA level in chocolate was found to be 23–537 μg/kg.
An ion trap liquid chromatography coupled to electrospray ionization tandem mass spectrometry method for the trace level determination of AA in bakery products was reported by Claus et al. (2005). Concentration of the analyte using a solid-phase-supported liquid–liquid extraction with ethyl acetate combined with the separation and drying of crumbs allowed the quantification of AA at levels ≥10.0 μg/kg. A LC-MS-MS method was used for confirmation and quantification of AA in infant rice cereals and other cereal-based foods by Zhang et al. (2005). The AA levels in infant rice cereals and other cereal-based foods were 3.3–37.1 and 10.9–1,568.9 μg/kg, respectively.
A LC-MS-MS method was reported for the trace quantitative analysis of AA in infant powdered milk and baby foods in jars by Jiao et al. (2005). The AA level in infant powdered milk and baby foods in jars were found to be 3.01–9.06 and 6.80–124.9 μg/kg, respectively. Another LC-MS-MS method was established for the determination of AA in food by Liu et al. (2006). The detection limit of the method was 2 μg/kg.
LC-ESP(electrospray)-MS-MS was applied for the confirmatory detection of AA (Yusa et al. 2006). Using the method, 62 samples of potato chips, snacks, biscuits, breakfast cereals, and crisp bread sampled from Valencia, Spain, supermarkets were analyzed. The levels (ranging from 11 to 2,880 μg/kg) were found to be similar to those reported in the European Union and the USA.
An optimization of a LC-MS-MS method was applied for the determination of AA at low levels in foods (mainly in potato and cereal products) with the purpose of routine analysis (Govaert et al. 2006). The LC-MS-MS method was supplied on the website of the US Food and Drug Administration. Due to a lack of sensitivity for certain foodstuffs with low AA content further tests were performed to optimize the method in terms of analytical performance. Its LOQ was found to be 20 μg/kg. AA contents of commercial market-purchased foods and of traditional home-cooked Korean foods were investigated using the LC-MS-MS method by Koh (2008). The effect of cooking method on AA amounts in potatoes was also studied. The extraction, purification, and determination of AA were according to the FDA method. According to the results, pre-treatment such as grinding, boiling, freezing, and thawing increased the AA formation in oil-fried potato. Temperature was more influential than time on AA formation during deep-oil frying. Removing water-soluble fractions reduced AA content of cooked potatoes.
A LC-MS-MS method for the simultaneous determination of acrylamide, asparagine, fructose, glucose, and sucrose in bread was developed by Nielsen et al. (2006). Recoveries were in the range 93–112% for AA spiked at 30 and 250 μg/kg and 97–101% for asparagine spiked at 70 and 140 mg/kg. A generic sample preparation method for the determination of AA in foods was developed with the LC-MS method by Gökmen and Şenyuva (2006). The LOD and LOQ were 2 and 6 ng/g, respectively.
Another analytical method based on LC-MS-MS for the determination of AA in foodstuffs was developed by Bermudo et al. (2006a, b). Atmospheric pressure chemical ionization as ionization source and an ion trap analyzer were used. Different levels of AA were obtained and pastry and dried fruits showed the lower levels (<20 ng/g). Potato chips and French fries gave values of the order of 500–9,250 ng/g. Another LC-MS-MS method was developed for the analysis of AA in processed foods using both internal and recovery standards (Kim et al. 2007). The AA content in the five foods (bread, candy, coffee, biscuits, and potato chips) ranged from 33 to 1,377 μg/kg.
A method for the analysis of AA in food using LC-MS-MS was performed to meet the demands of a collaborative validation trial (Rosen et al. 2007). A number of different matrices (e.g., mashed potato, coffee, and cereals) were spiked at levels from 5 to 4,000 μg/kg. Proficiency tests (n = 10) organized by Food Analysis Performance Assessment Scheme (FAPAS) and JRCIRMM proved that the method is reliable and has a broad range of applications.
An ultra-performance liquid chromatography coupled to ESP-MS-MS was reported for the determination of AA in potato crisps (Zhang et al. 2007a, b). This method allowed rapid quantitative determination of AA with a run time of only 3 min. The method validation data and proficiency test results (Z score, −0.1) of the official FAPAS suggested that the quantitative method could be applied for the rapid determination of AA in many investigations.
A modified sample preparation for AA determination in difficult matrices such as cocoa and coffee by a LC-MS-MS was described by Arisseto et al. (2008). For the identification and confirmation of AA, relative retention time and two diagnostic ions were monitored. Several typical foods produced and consumed in Spain were analyzed using LC-MS-MS in order to determine AA levels (Bermudo et al. 2008). A previously published LC-MS-MS method (Bermudo et al. 2006a, b) was used. Ion trap and time-of-flight mass analyzers were combined in order to characterize unequivocally an interfering co-extractive that was particularly present in potato-derived products. For that study, christmas sweets, olives, traditionally made potato crisps, pastry products, sweet fritters (“churros”), and one of Spain’s most famous dishes, Spanish omelet, were selected. The highest values were found in potato products and sweet fritters,
A LC-MS-MS method was developed in China to analyze AA in six tea varieties including green tea, oolong tea, black tea, white tea, yellow tea, and, Pu-erh tea (Liu et al. 2008). High AA levels occurred in baked, roasted, and one sun-dried green tea sample (46–94 ng/g), probably because temperatures between 100 °C and 150 °C were usually used during processing.
A collaborative trial tested an isotope dilution liquid chromatographic method with positive ESP-MS-MS for the analysis of acrylamide in bakery goods and potato products which was extended to the determination of acrylamide in roasted chestnuts and chestnut-based foods. As chestnuts have similar composition to potatoes, considerable amounts of acrylamide can be expected, especially in roasted chestnut products. The extended method was applied to 31 different chestnut samples (fresh, roasted, flour, cooked, glazed) that had been collected in nine European countries during 2005/2006 (Karasek et al. 2009). The influence of roasting time on the acrylamide content was also investigated in this study. The results showed that AA contents in roasted chestnuts could be quite high and chestnut-based products did not contain high levels of AA. The results of an exposure assessment of adolescents from Brazil to AA via selected foods was reported using an accredited LC-MS-MS method (Arisseto et al. 2009). The mean dietary intake was estimated to be 0.12 mg/kg bw/day, which is lower than the intakes reported for adolescents from other countries. The method was based on aqueous extraction of the roasted coffee matrix and solid-phase extraction (SPE) clean-up followed by isotope dilution LC-MS-MS by collaborative trial (Wenzl et al. 2009). Three coffee samples and one aqueous AA standard solution were sent to 11 laboratories from eight European Union Member Nations. Nine laboratories from eight countries reported analytical results for roasted coffee test samples covering the AA content range of 160–585 μg/kg.
Immunoassay
A study conducted by Preston et al. (2008) reported an immunoassay capable of detecting AA in spiked water samples with enzyme-linked immunosorbent assay (ELISA)-based detection system. The LOD is 65.7 μg/kg. In another study, AA in potato fries and biscuits were determined by a biotin–avidin enzyme-linked immunosorbent assay (BA-ELISA), with a working range of 10–100,000 ng/mL and a detection limit of 6 ng/mL (Zhou et al. 2008). According to the articles; future development of these assays will increase sensitivity further and immunoassay will be very useful for monitoring AA in food samples.
Conclusion
In this paper, analytical methods used for the determination of AA in food items are reviewed in detail.
Researchers are trying to improve the efficiency of analytical methods by focusing on sample treatment procedures which would enable implementation of only one analytical protocol to cover a wide range of food matrices, to simplify and to speed up sample preparation and cleaning, to decrease the LOD and LOQ, and considering economical and environmental issues, to decrease solvent use for analysis.
Because of its high solubility in water and its high reactivity and also because of the lack of a chromophor, AA is not easy to detect. UV detection coupled with chromatographic methods working at low wavelength (about 200 nm) have been developed. However, these methods are not selective enough for the analysis of AA in processed foods at very low levels. Thus, MS coupled to CE, GC, or LC is now the most widely used technique, for the determination of AA in foods. Analysis of AA in food products involves essentially chromatographic methods like GC-MS (derivatization of AA needed), LC-MS, and LC-MS-MS. LC-MS-MS is the most preferred. Typically, solid-phase extraction was used as sample preparation technique in these methods.
Public concern over AA contamination in food products, the aforementioned disadvantages of commonly implemented analytical methods and an increasing need for simple, rapid, precise, and time- and cost-effective determinations of acrylamide in food products are driving directed analytical chemists to focus on the development of new methods appropriate for screening this neurotoxic and possibly carcinogen compound.
References
Alves RC, Soares C, Casal S, Fernandes JO, Beatriz M, Oliveira PP (2010) Food Chem 119(3):929
Arisseto AP, Toledo MCF, Govaert Y, Loco JV, Fraselle S, Degroodt JM (2008) Food Anal Methods 1:49
Arisseto AP, Toledo MCF, Govaert Y, Loco JV, Fraselle S, Degroodt JM, Caroba DCR (2009) LWT—Food Sci Tech 42:207
Başkan S, Erim FB (2007) Electrophoresis 28:4108
Bermudo E, Ruiz-Calero V, Puignou L, Galceran MT (2004) Electrophoresis 25:3257
Bermudo E, Moyano E, Puignou L, Galceran MT (2006a) Anal Chim Acta 559:207
Bermudo E, Nunez O, Puignou L, Galceran MT (2006b) J Chromatogr A 1129:129
Bermudo E, Nunez O, Moyano E, Puignou L, Galceran MT (2007) J Chromatogr A 1159:225
Bermudo E, Moyano E, Puignou L, Galceran MT (2008) Talanta 76:389
Calbiani F, Careri N, Elviri L, Mangia A, Zagnoni I (2004) J AOAC Int 87(1):107
Chen B, Liu HZ, Yu P, Zhao JY, Chen X (2009) Food Sci Biotechnol 18:895
Claus A, Weisz GM, Kammerer DR, Carle R, Schieber A (2005) Mol Nutr Food Res 49:918
Croft M, Tong P, Fuentes D, Hambridge T (2004) Food Addit Contam 21:721
Dunovska L, Cajka T, Hajslova J, Holadova K (2006) Anal Chim Acta 578:234
Eberhart BL, Ewald DK, Sanders RA, Tallmadge DH, Zyzak DV, Strothers MA (2005) J AOAC Int 88(4):1205
ECHA (European Chemicals Agency) (2010) www.echa.europa.eu Accessed 30 March 2010
European Commission (2001) Scientific committee on food. Opinion on the Results of the Risk Assessment of Acrylamide, Brussel, March 2001
FAO/WHO (2002) Health implications of acrylamide in food. Report of a joint FAO/WHO consultation. WHO, Geneva
FDA (US Food and Drug Administration) (2006) www.cfsan.fda.gov Accessed 20 October 2009
Fennell TR, Sumner SC, Snyder RW, Burgess J, Friedman MA (2006) Toxicol Sci 93:256
Fernandes JO, Soares C (2007) J Chromatogr A 1175:1
Fohgelberg P, Rosen J, Hellenas KE, Abramsson ZL (2005) Food Chem Toxicol 43:951
Geng Z, Jiang R, Chen M (2008) J Food Compos Anal 21:178
Gökmen V, Şenyuva HZ (2006) J Chromatogr A 1120:194
Gökmen V, Şenyuva HZ, Acar J, Sarıoğlu K (2005) J Chromatogr A 1088:193
Goldmann T, Perisset A, Bertholet MC, Stadler RH, Petersson EV, Hellenäs KE (2006) Food Addit Contam 23:437
Govaert Y, Arisseto A, Van Loco J, Scheers E, Fraselle S, Weverbergh E, Degroodt JM, Goeyens L (2006) Anal Chim Acta 556:275
Granby K, Fagt S (2004) Anal Chim Acta 520:177
Granvogl M, Schieberle P (2006) J Agric Food Chem 54:5933
Granvogl M, Jezussek M, Koehler P, Schieberle P (2004) J Agric Food Chem 52:4751
Hashimoto K, Tanii H (1985) Mutat Res 158:129
Hoenicke K, Gatermann R, Harder W, Hartig L (2004) Anal Chim Acta 520:207
International Agency for Research on Cancer (IARC) (1994) Monographs on the evaluation of carcinogen risk to humans, 60, Lyon, France, p 389
Jiao J, Zhang Y, Ren Y, Wu X, Zhang Y (2005) J Chromatogr A 1099:198
Kaplan O, Kaya G, Ozcan C, Ince M, Yaman M (2009) Microchem J 93(2):173
Karasek L, Wenzl T, Anklam E (2009) Food Chem 114:1555
Kim CT, Hwang ES, Lee HJ (2007) Food Chem 101:401
Koh BK (2008) J Sci Food Agric 86:2587
Lee MR, Chang LY, Dou J (2007) Anal Chim Acta 582:19
LeQuesne PM (1980) Specific environmental neurotoxins. In: Spencer PS, Schaumburg HH, Williams, Wilkins (eds) Experimental and clinical neurotoxicology, 1st edn. Baltimore, MD, p 309
Liu HH, Chen CX, Liu QF, Liu XL, Yin JW (2006) Chin J Anal Chem 34:235
Liu J, Zhao G, Yuan Y, Chen F, Hu X (2008) Food Chem 108:760
Mottram DS, Wedzicha BL, Dodson AT (2002) Nature 419:448
NFA (Swedish National Food Administration) (2002) www.slv.se Accessed 26.05.2009
Nielsen NJ, Granby K, Hedegaard RV, Skibsted LH (2006) Anal Chim Acta 557:211
Paleologos EK, Kontominas MG (2005) J Chromatogr A 1077:128
Paulsson B, Kotova N, Grawé J, Henderson A, Granath F, Golding B, Törnqvist M (2003) Mutat Res 535:15
Preston A, Fodey T, Elliot C (2008) Anal Chim Acta 608(2):178
Ren Y, Zhang Y, Jiao J, Cai Z, Zhang Y (2006) Food Addit Contam 23(3):228
Rosen J, Nyman A, Hellenas KE (2007) J Chromatogr A 1172:19
Rufián-Henares JA, Morales FJ (2006) Food Chem 97:555
Soares CMD, Fernandes JO (2009) Food Anal Methods 2:197
Soares C, Cunha S, Fernandes J (2006) Food Addit Contam 23(12):1276
Soares CMD, Alves RC, Casal S, Beatriz M, Oliveira PP, Fernandes JO (2010) J Food Sci 75:57
Stadler RH, Blank I, Varga N, Robert F, Guy PA, Robert NC, Reidiker S (2002) Nature 419:449
Sumner SCJ, MacNeela JP, Fennell TR (1992) Chem Res Toxicol 5:81
Svensson K, Abramsson L, Becker W, Glynn A, Hellenäs KE, Lind Y, Rosén J (2003) Food Chem Toxicol 41:1581
Tezcan F, Erim FB (2008) Anal Chim Acta 617:196
Tsutsumiuchi K, Hibino M, Kambe M, Oishi K, Okada M, Miwa J, Taniguchi H (2004) J Food Hyg Soc Jpn 45(2):95
US Environmental Protection Agency (EPA) (1994) Chemical summary for acrylamide. Office of Toxic Substances, Washington DC
Wang H, Lee AWM, Shuang S, Choi MMF (2008) Microchem J 89:90
Wenzl T, Karasek L, Rosen J, Hellenaes KE, Crews C, Castle L, Anklam E (2006) J Chromatogr A 1132:211
Wenzl T, Szilagyi S, Rosen J, Karasek L (2009) Food Addit Contam 26(8):1146
Yusa V, Quintas O, Pardo O, Marti P, Pastor A (2006) Food Addit Contam 23(3):237
Zhang Y, Jiao J, Ren Y, Wu X, Zhang Y (2005) Anal Chim Acta 551:150
Zhang Y, Dong Y, Ren Y, Zhang Y (2006) J Chromatogr A 1116:209
Zhang Y, Jiao J, Cai Z, Zhang Y, Ren Y (2007a) J Chromatogr A 1142:194
Zhang Y, Ren Y, Zhao H, Zhang Y (2007b) Anal Chim Acta 584:322
Zhou X, Fan LY, Zhang W, Cao CX (2007) Talanta 71:1541
Zhou S, Zhang C, Wang D, Zhao M (2008) Analyst 133(7):903
Zhu Y, Li G, Duan Y, Chen S, Zhang C, Li Y (2008) Food Chem 109:899
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kepekci Tekkeli, S.E., Önal, C. & Önal, A. A Review of Current Methods for the Determination of Acrylamide in Food Products. Food Anal. Methods 5, 29–39 (2012). https://doi.org/10.1007/s12161-011-9277-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9277-2