Abstract
Sequential injection chromatography for the determination of benzoic acid (BA), sorbic acid (SOA), and salicylic acid (SA) has been investigated. Separation was performed on a monolithic C-18, (5 × 4.6 mm) column which is normally used as a guard column, with 1% acetonitrile: ammonium acetate buffer pH 4.5 as a mobile phase at a flow rate of 1.20 mL/min at ambient temperature and with the UV detection at 235 nm. Under the conditions, separation of the three compounds was achieved within less than 3 min. Linear calibrations were found to be 1–100 mg/L for the three: BA, SOA, and SA with detection limits of 1.9, 0.7, and 0.3 mg/L. The developed procedure was demonstrated to be an effective alternative fast and simple method for the analysis of food, fruit juice, syrup, and soft drink samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benzoic acid (BA) and sorbic acid (SOA) are widely used as acceptable food preservatives, if being added at a lower permitted amounts, i.e., BA of less than 1,000 mg/kg in fat emulsion food or fruit juice, less than 600 mg/kg in soft drink and SOA of not more than 1,000 mg/kg in fat emulsion food or fruit juice by the Codex General Standard for Food Additives and Thailand Food and Drug Administration. (FAO and WHO 2009; Food and Drug Administration, Thailand 2008) However, salicylic acid (SA), a dermato-fugicidal substance, which is not a food preservative and not at all being allowed to be added in food, is illegally used in foods and beverages due to its superior effectiveness in controlling mold and inhibiting yeast growth as in Thailand (Notification of Thailand Ministry of Public Health 1993). Since the maximum concentrations of the preservatives in each type of food are limited and it is essential to prevent the use of other alternative non-allowable substances in place of food preservatives, it is necessary to determine the levels of these preservatives in food to ensure that they are present in the permitted limits such as for BA and SOA but for SA, only qualitative analysis is needed, as SA is not at all allowed to be added in any food/beverages. Various analytical techniques have been reported for assaying BA and SOA. Conventional techniques such as titrimetry, spectrophotometry (Helrich 1990), and thin layer chromatography (Thomassin et al. 1997) usually require extensive sample-pretreatment, and in practice, the reliability of these traditional methods is low. Chromatographic techniques including high performance liquid chromatography (HPLC) (Ferreira et al. 2000; Mikami et al. 2002; Saad et al. 2005; Techakriengkrai and Surakarnkul 2007; Tfouni and Toledo 2002; Marjan et al. 2010), gas chromatography (Dong and Wang 2006; Wang et al. 2006), and capillary electrophoresis (CE) (Costa et al. 2008; Han et al. 2008; Tang and Wu 2007; Waldron and Li 1996) are the most common for the detection and quantification of these preservatives in foods and beverages. Among them, HPLC has been mainly employed, due to good separation efficiency and sensitivity. However, HPLC may not be made available in some places due to its cost.
Sequential injection chromatography (SIC) was introduced (Chocholous et al. 2007) by employing a short monolithic column composed of a single piece of porous silica gel but having high performance possibly equivalent to a typical C-18 5-μm particulate HPLC conventional column (Satinsky et al. 2004) SIC system offers miniaturization, rapidity, high sensitivity, and waste reduction. It involves low usage of organic solvent as compared to HPLC. The early works of SIC analysis were reported on the analysis of relatively simple multi-component samples such as pharmaceuticals. So far, SIC has not been applied to food and beverage analysis.
Here, we present a simple SIC employing monolithic column for a simple and fast method for the analysis of SA, BA, and SOA in food, fruit juice and syrup, and soft drink samples. This could be alternative to the existing procedures.
Material and Methods
Chemicals and Reagents
All chemicals used were of analytical reagent grade unless otherwise stated. Ultrapure water (obtained form a system of Milli-Q, Millipore, Sweden) was used throughout the whole study. Stock standard solutions of SA, BA, and SOA (all of Merck, Germany) were prepared at 1,000 mg/L each by dissolving 0.025 g of each compound in mobile phase, and the solution was stored at 4 °C. Working standard solutions were daily prepared by appropriate diluting the stock standard solutions with mobile phase. The mobile phase was 1% acetonitrile (HPLC grade, LAB-SCAN, Ireland) and 0.01 M ammonium acetate buffer pH 4.5. An ammonium acetate buffer (0.01 M, pH 4.5) was prepared by dissolving 0.078 g of ammonium acetate dehydrate (AR, Carlo Erba, Italy) in water, and its pH was adjusted to 4.5 with 0.01 M acetic acid (Carlo Erba, Italy). Then, the final volume was made up to 100 ml with water. The mobile phase was filtered through a 0.45-μm filter membrane and was degassed by placing it in an ultrasonic bath.
Preparation of Samples
Food samples which are not in liquid/solution form such as mayonnaise, jam, Thai custard were treated to be slurry with fine particles prior to the extraction by water. Each sample was mixed with water at a ratio of 5-g sample: 10 mL water, and then the mixtures were shaken by hand for about 1 min. The suspensions were heated in a water bath at 80 °C for 15 min. Then, they were filtered through filter papers (Whatman no.1), and the filtrates were diluted with water to the appropriate volumes.
Liquid samples such as fruit juice, syrup, and soft drinks were centrifuged at 2,000 rpm for 10 min. A 1-mL aliquot of the supernatant from each sample was diluted with appropriate volume of water. In order to extend the column life, it should be more convenient to use a 0.45-μm filter (cellulose acetate membrane (Tfouni and Toledo 2002)).
Instrument and Apparatus
SIC system was assembled as illustrated in Fig. 1. A C18 monolithic column (Chromolith® Flash RP-18e 5 × 4.6 mm, Merck), commonly used as a guard column in HPLC system, was employed as a mini-analytical column to perform the separation of SA, BA, and SOA. A 5-ml syringe pump and a 10-port multi-position valve (Valco Instrument Co., USA) were used for aspiration of solutions into the system. The holding coil was made of PTFE tubing (1.6 mm i.d., 1.0-m long, and 2 mL capacity). Other flow lines were made of a PTFE tubing of 0.8 mm. i.d. The operational steps of the SIC system was controlled by a computer system using an in-house developed program written in Visual Basic 6 (Microsoft, USA) (Siriangkhawut et al. 2009). A spectrometric detection was assembled from a UV-light source D-1000-CE (Analytical Instrument system Inc., Flemington, USA), fiber optic cables (P 200-2-UV/Vis, FIA lab instruments, USA), a Z-flow cell (10-mm path length, Anates, CO, USA), and a diode array spectrometer (USB 2000, Ocean Optics Inc., Dunedin, USA). The FIAlab software version 5.0 was used for signal processing in the detection system.
SIC Operation
The operational sequences for the separation of SA, BA, and SOA by the SIC are given in Table 1. Before running the sequences, the holding coil and the tubing connecting to port 8 of the selection valve was filled with mobile phase. Other tubing which connected to other ports of the selection valve were filled with their designated solutions. Then, operational sequences were started. First, the mobile phase (3,000 μl) and sample solution (5 μl) were sequentially aspirated into the holding coil. Then, the zone was propelled through the Chromolith® reverse-phase column for separation. After that, the effluent was detected by UV-vis detector at 235 nm.
Results and Discussion
Optimization of the System
Detection Wavelength
The absorption spectra of SA, BA, and SOA were recorded from 200–400 nm. Absorption maxima for SA, BA, and SOA were found at 230, 226, and 258 nm, respectively, as shown in Fig. 2. For simultaneous detection, the wavelength of 235 nm was chosen to compromise the sensitivities of the three compounds.
It should be noted that by using a CCD detector, this could obtain better sensitivity in the measures by selecting the most sensitive wavelength for each analyte. For the same reason, probably they might improve the quality of the baseline using another wavelength in order to correct the refractive index (Schlieren effect).This should be further explored.
pH of Mobile Phase
The retention abilities of the three analytes were affected by pH of the mobile phase. The mobile phase of pH 4.5 was chosen, as based on the suitable working pH of the column (Ferreira et al. 2000). At this pH, SA (pKa = 3.0) and other acids (such as citric and ascorbic acids) would also become anion and would be eluted out first and therefore, would not affect the separation of BA and SOA. BA (pKa = 4.2) would also present as an anion and would be eluted before SOA (pKa = 4.8). Therefore, the elution order of the three compounds under the pH 4.5 was observed to be SA, BA, and then SOA.
Mobile Phase Composition
The ratio of organic solvent composition in mobile phase was studied. A series of acetonitrile (1%, 3%, 6%, and 9%) in 0.01-M ammonium acetate buffer pH 4.5 was studied. It was found that shorter retention times but lower resolution of SA, BA, and SOA were obtained with increasing ratio of organic solvent. Therefore, the 1% acetonitrile was chosen as compromising between retention time and the usage amount of organic composition.
Flow Rate
The flow rates of mobile phase were studied at 0.78, 1.02, 1.20, 1.50, and 1.80 mL/min. The results indicated that flow rate did not much affect the separation efficiency. However, a flow rate of 1.20 mL/min was chosen rather than at a higher flow rate to prevent overlapping of peaks in case of high concentrations of the analytes. At this flow rate, the separation was achieved within 3 min.
Characteristics and Figures of Merits of the SIC System
Under the selected operational conditions (column, Chromolith® Flash RP-18e length 5 mm, i.d. 4.6-mm column, mobile phase 1% acetonitrile: ammonium acetate(0.01 M) buffer pH 4.5, flow rate: 1.20 mL/min, injection volume: 5 μL, and detection wavelength: 235 nm), the calibration graphs and the basic chromatographic parameters were obtained. The analysis time for one separation was 2.5 min (leading to a sample throughput of 24 samples per hour). Each analysis cycle consumed 5 μL of sample, 3,000 μl of mobile phase (1% acetonitrile and the acetate buffer).
The SI chromatograms, as shown in Fig. 3 (shown up to 25 mg/L), resulted in the calibration graphs for 1–100 mg/L of SA, BA, and SOA with the following linear regression equations: y = 42.062x − 55.432 (R 2 = 0.9990), y = 43.019x − 70.842 (R 2 = 0.9981), and y = 54.511x − 116.58 (R 2 = 0.9975), respectively, where y is analytical signal (peak area, millivolts per second) and x is concentration (milligrams per liter) of the analyte. Other figures of merits were evaluated and summarized in Table 2.
It should be remarked that the order of elution of BA and SOA was similar to that obtained from a HPLC procedure (Tfouni and Toledo 2002) for a quince jam samples with a set of conditions employing a C18 analytical column of 30 cm, 20-μL injection volume, acetonitrile/water/acetate buffer pH 4.2 (17:81:2 v/v) mobile phase at a flow rate of 1.0 mL/min and 228 and 260-nm detection wavelength. The retention times of BA and SOA were 8 and 10.7 min, respectively. Recently, as isocratic operating HPLC produce using C18 column (4.6 × 250 mm) with 20-μL injection volume and a mobile phase of ammonium acetate buffer (pH 4.2): acetonitrile (80:20) with 0.8 mL/min; 225-nm detection provided the retention time of 6.4, 14.9, and 20.0 min for SA, BA, and SOA, respectively.
It can be then clearly marked that the retention times of BA and SOA obtained from the SIC system were dramatically reduced to 0.83 and1.5 min, respectively. The whole process of separation, including column equilibration, by the proposed SIC system was achieved in a much shorter analysis time (2.5 min) as compared to 15 min of that HPLC procedure. Other features compared between the HPLC procedures (Tfouni and Toledo 2002) and the proposed SIC system as shown in Table 3.
As citric acid and ascorbic acid may present in fruit juice and soft drink, therefore, the interference effects due to these two acids were studied. It was found that citric acid as high as 10 mg/L was not observed in the SIC chromatogram, while ascorbic acid (10 mg/L) was at void volume peak. This indicated that both the acids should not affect the analysis of BA and SOA.
Analysis of Real Samples
The developed system was applied for analysis of food preservatives (BA and SOA) in some food samples. These samples were collected from food sections in the department stores around Chiang Mai, Thailand.
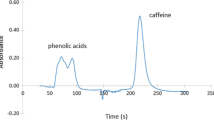
Figure 4 represents the examples of SI chromatograms obtained from a mixed standard solution, non-spiked mayonnaise, and non-spiked grape juice samples. To perform the recovery study, food and beverage samples were spiked with 20 μg/mL of mixed standard preservatives (BA and SOA). Table 4 summarizes the results. No SA was detected in the samples. Percent recoveries were found to be of 96–107% and 94–108% for BA and SOA, respectively.
SI chromatograms of a mixed standards of salicylic acid (SA), benzoic acid (BA), and sorbic acid (SOA) at 25 mg/L; b non-spiked mayonnaise sample; c non-spiked grape juice (condition, 5 μL injection volume, 1%acetonitrile/ammonium acetate buffer pH 4.5 (v/v) mobile phase at the flow rate of 1.20 mL/min and detection wavelength 235 nm)
From the foods and beverages taken as samples, it was found that the amounts of BA and SOA were within the permitted level by The Thailand Food and Drug Administration. No SA was detected in the samples.
Conclusion
SIC procedure has been proposed for the analysis of SA, BA, and SOA in food, fruit juice, syrup, and soft drink samples. Mobile phase was composed of less amount of organic solvent as compared to that used for the previously reported conventional HPLC systems. The benefits of the proposed SIC system includes short analysis time, miniaturization, low sample/mobile phase consumption, resulting in reduction of waste production, and instrumentation cost. The SIC system could be used as an alternative for rapid routine analysis of food and soft drink samples and is feasibly developed future to be a portable analyzer. This should be very useful for on-site analysis.
References
Chocholous P, Solich P, Satinsky D (2007) Anal Chim Acta 600(1–2):129
Costa ACO, da Silva Perfeito L, Tavares MFM, Micke GA (2008) J Chromatogr A 1204(1):123
Dong C, Wang W (2006) Anal Chim Acta 562(1):23
FAO and WHO (2009) GSFA Online Food Additive Group Details for Benzoates and Sorbates, Retrieved Mar 27, 2011 from: http://www.codexalimentarius.net/gsfaonline/groups/details.html?id=162 and http://www.codexalimentarius.net/gsfaonline/groups/details.html?id=10
Ferreira IMPLVO, Mendes E, Brito P, Ferreira MA (2000) Food Res Int 33(2):113
Food and Drug Administration, Thailand (2008) Retrieved Feb 15, 2009 from http://newsser.fda.moph.go.th/food/English_Version_Index.php
Han F, He YZ, Li L, Fu GN, Xie HY, Gan WE (2008) Anal Chim Acta 618(1):79
Helrich K (1990) Official methods of analysis of the Association of Official Analytical Chemists (AOAC) 1143
Marjan M, Zahra S, Gholamreza D, Katayoun J (2010) Food Anal Methods, online first on Jul 30, 2010
Mikami E, Goto T, Ohno T, Matsumoto H, Nishida M (2002) J Pharmaceut Biomed 28(2):261
Notification of Thailand Ministry of Public Health, No. 151 (1993) Published in the Royal Government Gazette, vol 111, part 9D, dated 4 February 1994
Saad B, Bari MF, Saleh MI, Ahmad K, Talib MKM (2005) J Chromatogr A 1073(1–2):393
Satinsky D, Neto I, Solich P, Sklenarova H, Conceicao M, Montenegro BSM, Alberto NA (2004) J Sep Sci 27(7–8):529
Siriangkhawut W, Pencharee S, Grudpan K, Jakmunee J (2009) Talanta 79(4):1118
Tang Y, Wu M (2007) Food Chem 103(1):243
Techakriengkrai I, Surakarnkul R (2007) J Food Compos Anal 20(3–4):220
Tfouni SAV, Toledo MCF (2002) Food Contr 13(2):117
Thomassin M, Cavalli E, Guillaume Y, Guinchard C (1997) J Pharmaceut Biomed 15(6):831
Waldron KC, Li J (1996) J Chromatogr B 683(1):47
Wang L, Zhang X, Wang Y, Wang W (2006) Anal Chim Acta 577(1):62
Acknowledgments
The authors thank the Commission on Higher Education (CHE) and The Thailand Research Fund (TRF) through Research grant for support. Additional partial support from the Center of Excellence for Innovation in Chemistry: Post Graduate Education and Research Program in Chemistry (PERCH-CIC) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jangbai, W., Wongwilai, W., Grudpan, K. et al. Sequential Injection Chromatography as Alternative Procedure for the Determination of Some Food Preservatives. Food Anal. Methods 5, 631–636 (2012). https://doi.org/10.1007/s12161-011-9276-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9276-3