Abstract
The aim of the present study was to find out the best method for extracting antioxidants from Syzygium cumini L. leaves. The extraction was done by three different methods: sequential cold percolation extraction method, decoction extraction method, and maceration extraction method. Antioxidant activity, total phenol, and flavonoid content were determined in all different extracts of various extraction methods of S. cumini L. leaves. Antioxidant activity was tested by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical, superoxide anion radical and hydroxyl radical scavenging activities, and reducing capacity assessment. Sequential cold percolation extraction method proved to be the best extraction method. The acetone extract had maximum phenol and flavonoid content and showed best DPPH free radical scavenging activity and reducing capacity assessment. Ethyl acetate extract showed best superoxide radical scavenging activity, while aqueous extract showed best hydroxyl radical scavenging activity. It can be concluded that sequential cold percolation extraction method is the best method of extracting leaf antioxidants for this plant at least.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free radicals have been shown to be harmful as they react with important cellular components such as proteins, DNA, and cell membrane (Mantena et al. 2008). All organisms contain an anti-free radical defense system, which includes antioxidant enzymes like catalase, peroxidase, and superoxide dismutase and antioxidants like ascorbic acid and tocopherols (Kalaivani and Mathew 2010). Oxidative stress is caused by an insufficient capacity of biological systems to neutralize excessive free radical production, which contribute to human diseases and aging (Flora 2007), including cardiovascular disease (Victor and Rocha 2007), neurodegenerative disease and age-related cognitive decline (Swerdlow 2007), obesity and insulin resistance (Martinez 2006), as well as immune system dysfunction (Larbi et al. 2007).
There are many synthetic antioxidants like butylated hydroxytoluene, butylated hydroxyanisole, propyl gallate, and tertbutyl hydroxytoluene in use, but many side effects are reported (Ito et al. 1983); therefore, there is a great demand and a need for natural antioxidants. Medicinal plants from time immemorial have been used in virtually all cultures as a source of medicine (Cragg and Newmann 2001). They are considered as the backbone of traditional medicine and are widely used to treat a plethora of acute and chronic diseases ranging from common cold to complex human diseases all over the world. From Aspirin to Taxol, modern pharmaceutical industries largely take profit of the diversity of secondary metabolites in medicinal plants for new drug research.
Some of the most common practices involve the use of crude plant extracts, which may contain a broad diversity of molecules with often unknown biological effects. Potential sources of antioxidant compounds have been looked for in several types of plant materials such as vegetables, fruits, leaves, oilseeds, cereal crops, bark and roots, spices herbs, and crude plant drugs (Ramarathnam et al. 1995). However, it has been reported that generally, leaves are selected for antioxidant studies (Chanda and Dave 2009).
Traditionally, medicinal plants are boiled in water (this extraction process is called the decoction method), and the extracts are consumed. In another extraction method called maceration method, the medicinal plants are soaked in ethanol or organic solvent for a definite period and then consumed. These methods have been commonly used for thousands of years. The extraction efficiencies of these methods of extracting antioxidants from medicinal plants are not known. In sequential cold percolation extraction method, the medicinal plants are kept in one solvent for 24 h and then filtrate is collected, and the same material is extracted in another solvent in a similar manner and no heat is provided (Baravalia et al. 2009). The advantage of this method is to get more yield of plant materials. The second advantage of the method is that it uses cold percolation process where plant material is left overnight in a solvent for complete extraction of plant material. The third advantage of sequential cold extraction method is use of solvents of different polarities starting from nonpolar to polar. Plant constituents will be dissolved in solvents according to their nature of solubility, e.g., lipid will dissolve in nonpolar solvents like toluene, while sugar, phenols, etc. will dissolve in polar solvents like acetone (Yang et al. 2007).
Syzygium cumini L. belonging to the family Myrtaceae is a tropical evergreen tree, originating from India and Sri Lanka, which usually reaches a height of 7–10 m. It has been attributed in the Indian folklore medicine system to possess several medicinal properties (Warrier et al. 1996). Different parts of this plant such as seeds, bark, fruits, and leaves have been used in traditional medicine as remedy for diabetes mellitus in many countries (Rahman and Zaman 1989; Teixeira et al. 1997). The fruit skin of S. cumini has significant antioxidant activity (Banerjee et al. 2005). The leaves of S. cumini have potential anti-inflammatory activity and were correlated with phenolic content (Lima et al. 2007). The plant possesses compounds like acetyl oleanolic acid, triterpenoids, ellagic acid, quercetin, kaempferol, and myricetin in different concentrations (Rastogi and Mehrotra 1990). This study was aimed to assess the feasibility of extracting antioxidant compounds from S. cumini L. leaf by different extraction methods. Generally, people use either one of these methods for any type of study, but we want to ascertain the best method for evaluation of antioxidant potential of this plant (irrespective of the method used).
Material and Methods
Collection of the Plant Material
The leaves of S. cumini were collected in October 2008 from semi-arid region of Jamnagar, Gujarat, India. The leaves were separated, washed thoroughly with tap water, shade dried, homogenized to fine powder, and stored in an airtight bottle.
Chemicals
Analytical grade of all chemicals and reagents were purchased: 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Sigma Chemical Co. (St Louis, MO, USA), hydrochloric acid (HCl) and other organic solvents were from MERCK Pvt. Ltd. (India), phenazine methosulphate (PMS), nicotinamide adenine dinucleotide reduced (NADH), nitroblue tetrazolium (NBT), and ferric chloride (FeCl3) were from Hi-media (India), and ethylene diaminetetraacetic acid (EDTA), trichloroacetic acid (TCA), thiobarbituric acid (TBA), and potassium ferricyanide (K3Fe(CN)6) were from Sisco Research Laboratories Pvt. Ltd. (India).
Decoction Extraction Method
For the decoction method (Li et al. 2007), 5 g of dried leaf powder was extracted with 100 ml of deionized water at 100 °C for 30 min in a water bath. The extract was filtered with eight layers of muslin cloth and centrifuged at 5,000 rpm for 10 min. The supernatant was collected, and the solvent was evaporated using a rotary vacuum evaporator (Equitron, India) to dryness. The extract was stored at 4 °C in an airtight bottle. The residue was weighed to obtain the extractive yield.
Maceration Extraction Method
For maceration method, 5 g of dried leaf powder was extracted with 100 ml of 50% aqueous ethanol at 25 °C for 42 h in static condition (An 2000). The extract was filtered with eight layers of muslin cloth and centrifuged at 5,000 rpm for 10 min. The supernatant was collected, and the solvent was evaporated using a rotary vacuum evaporator (Equitron, India) to dryness. The extract was stored at 4 °C in an airtight bottle. The residue was weighed to obtain the extractive yield.
Five grams of dried leaf powder was extracted with 100 ml of 80% aqueous methanol at 35 °C for 24 h in an incubator (Cai et al. 2004). The extract was filtered with eight layers of muslin cloth and centrifuged at 5,000 rpm for 10 min. The supernatant was collected, and the solvent was evaporated using a rotary vacuum evaporator (Equitron, India) to dryness. The extract was stored at 4 °C in an airtight bottle. The residue was weighed to obtain the extractive yield.
Sequential Cold Percolation Extraction Method
For sequential cold percolation extraction method (Wiart et al. 2004; Parekh and Chanda 2007), 10 g of dried leaf powder was taken in 100 ml of petroleum ether in a conical flask, plugged with cotton wool, and then kept on a rotary shaker at 120 rpm for 24 h. After 24 h, the extract was filtered with eight layers of muslin cloth and centrifuged at 5,000 rpm for 10 min. The supernatant was collected, and the solvent was evaporated using a rotary vacuum evaporator (Equitron, India) to dryness. The residue was then taken successively in 100 ml of solvent (toluene, ethyl acetate, acetone, and water) and was kept on a rotary shaker at 120 rpm for 24 h. Then, the procedure followed was same as above, and the dry extract was stored at 4 °C in airtight bottles. The residues were weighed to obtain the extractive yield.
Determination of Total Phenol Content
The amount of total phenol content was determined by Folin-Ciocalteu’s reagent method (Mc Donald et al. 2001). The extract (0.5 ml) and 0.1 ml of Folin-Ciocalteu’s reagent (0.5 N) were mixed, and the mixture was incubated at room temperature for 15 min. Then, 2.5 ml of saturated sodium carbonate solution was added and further incubated for 30 min at room temperature, and the absorbance was measured at 760 nm using a digital spectrophotometer (Systronic, India), against a blank sample. The calibration curve was made by preparing gallic acid (10 to100 μg ml−1) solution in distilled water. Total phenol content is expressed in terms of Gallic acid equivalent (milligrams per gram of extracted compounds).
Determination of Total Flavonoid Content
The amount of flavonoid content was determined by aluminium chloride colorimetric method (Chang et al. 2002). The reaction mixture (3.0 ml) consisted of 1.0 ml of sample (1 mg ml−1) and 0.5 ml of aluminium chloride (1.2%) and 0.5 ml potassium acetate (120 mM) and was incubated at room temperature for 30 min. The absorbance of all samples was measured at 415 nm using a digital spectrophotometer (Systronic, India), against a blank sample. The calibration curve was made by preparing a quercetin (5 to 60 μg ml−1) solution in methanol. The flavonoid content is expressed in terms of standard equivalent (milligrams per gram of extracted compound).
Determination of DPPH Free Radical Scavenging Activity
DPPH free radical scavenging activity was measured by the modified method of McCune and Johns (2002). The reaction mixture (3.0 ml) consisted of 1.0 ml DPPH in methanol (0.3 mM), 1.0 ml methanol, and 1.0 ml of different concentrations (5 to 140 μg ml−1) of different solvent extracts diluted by methanol. The mixture was incubated in dark for 10 min, after which the absorbance was measured at 517 nm using a digital spectrophotometer (Systronic, India), against a blank sample. Ascorbic acid (2 to 16 μg ml−1) was used as positive control (Liu et al. 2011). Percentage of inhibition was calculated using the following formula:
where A 0 is the absorbance of control and A 1 is the absorbance of test.
Determination of Superoxide Radical Scavenging Activity
The superoxide radical scavenging activity was measured as described by Robak and Gryglewski (1988). Superoxide radicals are generated by oxidation of NADH and assayed by the reduction of NBT. The reaction mixture (3.0 ml) consisted of 1.0 ml of different concentrations (50 to 500 μg ml−1) of different solvent extracts diluted by distilled water, 0.5 ml Tris–HCl buffer (16 mM, pH 8), 0.5 ml NBT (0.3 mM), 0.5 ml NADH (0.93 mM), and 0.5 ml PMS (0.12 mM). The superoxide radical generating reaction was started by the addition of PMS solution to the mixture. The reaction mixture was incubated at 25 °C for 5 min, and then, the absorbance was measured at 560 nm using a digital spectrophotometer (Systronic, India), against a blank sample. Gallic acid (50 to 225 μg ml−1) was used as a positive control (Robak and Gryglewski 1988). Percentage of inhibition was calculated using the formula described as above.
Determination of Hydroxyl Radical Scavenging Activity
The hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and test compound for hydroxyl radical generated by Fe+3–Ascorbic acid–EDTA–H2O2 system (Fenton reaction) according to the method of Kunchandy and Rao (1990). The reaction mixture (1.0 ml) consisted of 0.1 ml 2-deoxy-2-d-ribose (28 mM in 20 mM KH2PO4–KOH buffer, pH 7.4), 0.5 ml of the different concentrations (200 to 1,000 μg ml−1) of solvent extracts diluted by distilled water, 0.2 ml EDTA (1.04 mM), and 200 μM FeCl3 (1:1 v/v), 0.1 ml H2O2 (1.0 mM), and 0.1 ml ascorbic acid (1.0 mM) was incubated at 37 °C for 1 h. The 1.0 ml TBA (1%) and 1.0 ml TCA (2.8%) were added and incubated at 100 °C for 20 min. After cooling, absorbance of pink color was measured at 532 nm using a digital spectrophotometer (Systronic, India), against a blank sample. Gallic acid (20 to 200 μg ml−1) was used as a positive control (Kunchandy and Rao 1990). Percentage of inhibition was calculated using the formula described as above.
Reducing Capacity Assessment
The reducing capacity assessment of different solvent extracts of leaves was determined using the modified method of Athukorala et al. (2006). One milliliter of different concentrations (20 to 180 μg ml−1) of different solvent extracts diluted by distilled water was mixed with 2.5 ml phosphate buffer (200 mM, pH 6.6) and 2.5 ml K3Fe(CN)6 (30 mM). The mixture was then incubated at 50 °C for 20 min. Thereafter, 2.5 ml of TCA (600 mM) was added to the reaction mixture and then centrifuged for 10 min at 3,000 rpm. The upper layer of solution (2.5 ml) was mixed with 2.5 ml distilled water and 0.5 ml FeCl3 (6 mM), and the absorbance was measured at 700 nm using a digital spectrophotometer (Systronic, India), against a blank sample. Ascorbic acid (20 to 180 μg ml−1) was used as positive control (Ksouri et al. 2009).
Results and Discussion
Antioxidants have been defined as substances that, when present at low concentration compared with oxidizable compounds (e.g., DNA, protein lipids, or carbohydrate), delay or prevent oxidative damage due to the presence of reactive oxygen species (ROS). These ROS undergo redox reaction with phenolic, resulting in inhibition of antioxidant activity in a concentration-dependent manner (Halliwell and Gutteridge 1990). Thus, measurement of total phenols and antioxidant activity has increasingly been used in plant samples and has become an important tool for investigation.
Extractive Yield
The extraction yield depends on solvents, time and temperature of extraction, as well as the chemical nature of the sample. Under the same time and temperature conditions, the solvent used and the chemical property of sample are the two most important factors (Shimada et al. 1992). The extractive yield obtained by various extraction methods is shown in Fig. 1. The extractive yield varied among different solvents. In sequential cold percolation method, aqueous extract (aqueous 1) showed highest extractive yield than other organic solvents. In the other two extraction methods, aqueous extract (aqueous 2) of decoction extraction method showed highest yield as compared to the other two extracts of maceration method (50% ethanol and 80% methanol).
It was noted that the extraction efficiency achieved by using boiling water (decoction method) was much greater than that achieved by using maceration method and sequential cold percolation extraction method. The higher temperature led to an improved extraction efficiency, but it did not seem to have a significant negative effect on the antioxidant capacities of the phenolic compounds extracted because only a short heating time was used.
Total Phenol and Flavonoid Content
Polyphenols have many favorable effects on human health like inhibiting the oxidation of low-density proteins (Frankel et al. 1993), thereby decreasing the risk of heart diseases (Williams and Elliots 1997). They have anti-inflammatory and anticarcinogenic properties (Carrol et al. 1999; Maeda-Yamamoto et al. 1999). Also, flavonoids and many other phenolic compounds of plant origin have been reported as scavenger of reactive oxygen species (ROS) and are viewed as promising therapeutic drugs for free radical pathogens (Lee et al. 2000). Thus, measurements of polyphenols and antioxidant activity in herbs have become important tools to understand the reactive values of plant species (Amarowicz et al. 2010) from a health point of view.
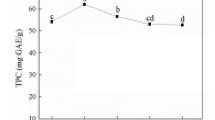
Total phenol and flavonoid contents of all extraction methods are shown in Fig. 2. The total phenol content was more than the total flavonoid content in all the extraction methods. In sequential cold percolation method, acetone extract showed highest content of phenol as compared to other solvent extracts, while in the other two extraction methods (decoction and maceration method), the three extracts (aqueous 2, 50% ethanol and 80% methanol) showed almost similar phenol content. When both aqueous extracts are compared, cold percolation aqueous extract (aqueous 1) showed slightly low total phenolic content as compared to decoction aqueous extract (aqueous 2). The flavonoid content was more in all the extracts extracted in sequential cold percolation extraction method; unlike total phenolic content, the flavonoid content of aqueous extract of sequential cold percolation extraction method was more than the content from the decoction method. Maximum phenol and flavonoid contents were present in acetone extract, but Kaneria et al. (2009) reported maximum total phenol content in methanol extract of S. cumini; the contradictory results may be because of the different extraction methods used by Kaneria et al. (2009).
Both phenol and flavonoid contents were minimum in toluene extract. It is generally believed that plants which are having more phenolic content show good antioxidant activity, and that is there is a direct correlation between total phenol content and antioxidant activity (Gholivand et al. 2010; Chanda and Nagani 2010; Rakholiya et al. 2011). It can be stated that phenolic content of the plant may be a good indicator of its antioxidant capacity.
DPPH Free Radical Scavenging Activity
Several methods have been used to measure free radical scavenging capacities of plant. The DPPH radical scavenging activity has been widely used as a model system to investigate the scavenging activity of natural compounds. In the present study, all the samples, irrespective of the solvents used or the different extraction methods, showed scavenging activity in a concentration-dependent manner.
In DPPH free radical scavenging assay, toluene extract showed IC50 value more than 1,000 μg ml−1, while other extracts showed varied levels of DPPH radical scavenging activity. Acetone extract showed best DPPH radical scavenging activity (IC50 = 20 μg ml−1) in comparison to other organic solvents and aqueous extract (Table 1). When aqueous extracts of both extraction methods were compared, aqueous extract of sequential cold percolation extraction method showed better DPPH activity (Table 1). The IC50 value of aqueous extract of sequential cold percolation extraction method was 34.5 μg ml−1 while aqueous extract of decoction method was 60.5 μg ml−1. It appears that sequential cold percolation extraction method is best for DPPH free radical scavenging activity. This might be due to the different polarities of solvents used and the chemical property of the sample. The acetone extract showed lowest IC50 value (20 μg ml−1) followed by 50% ethanol extract (maceration method) (IC50 = 40.4 μg ml−1; Table 1).
In the present study, it was observed that the greatest antioxidant activity had a direct correlation with quantities of total phenols both in aqueous as well as organic solvent extracts. Similar findings have been reported earlier (Brighente et al. 2007; Ruan et al. 2008; Chanda et al. 2010), suggesting a causative relationship between total phenol content and antioxidant activity indicating that the phenolic compound present in the plant S. cumini may be responsible for the antioxidant properties.
Superoxide Anion Radical Scavenging Activity
Although superoxide anion is a weak oxidant, it gives rise to generation of powerful and dangerous hydroxyl radical as well as singlet oxygen, both of which contribute to oxidative stress (Meyer and Isaksen 1995).
Except for 80% methanol extract, all the other seven extracts showed good activity. Amongst the three extraction methods, in sequential cold percolation extraction method, the ethyl acetate extract showed highest activity (IC50 = 157 μg ml−1) as compared to other organic solvent extracts (Table 1), while in maceration method, 50% ethanol (Table 1) showed better activity than aqueous extract by decoction method (Table 1). When two aqueous extracts are compared, aqueous extract by sequential cold percolation extraction method (Table 1) showed better activity than aqueous extract by decoction extraction method (Table 1).
Hydroxyl Radical Scavenging Activity
The hydroxyl radical is one of the potent reactive oxygen species in the biological system. It reacts with the polyunsaturated fatty acid moieties of cell membrane, phospholipids, and causes damage to cell (Halliwell and Gutteridge 1981). When hydroxyl radical scavenging activity by different methods of extraction is compared, the aqueous extract (IC50 = 500 μg ml−1) of sequential cold percolation extraction method showed highest activity followed by 80% methanolic extract (IC50 = 600 μg ml−1) by maceration extraction method. The aqueous extract (IC50 = 700 μg ml−1) of decoction method extraction showed less activity as compared to the other two methods. All other organic solvent extracts of the three different extraction methods showed IC50 value more than 1,000 μg ml−1 (Table 1). It can be stated that the polar solvents lead to improved extraction efficiency and showed good hydroxyl radical scavenging activity.
Reducing Capacity Assessment
In the reducing power assay, the presence of antioxidants in the extract results in the reduction of Fe3+ to Fe2+ by donating an electron. The amount of Fe2+ can then be monitored by measuring the formation of blue color at 700 nm. Increasing absorbance indicates an increase in reductive ability. The results show that there was an increase in reducing power of the extract as the extract concentration increases.
All the extracts showed different levels of reducing capacity. Acetone extract by sequential cold percolation extraction method showed maximum absorbance and hence maximum reducing capacity assessment among various solvents extracts (Fig. 3). The reducing capacity assessments of other extracts were in the order: acetone extract by sequential cold percolation extraction method <80% methanol by maceration extraction method <50% ethanol by maceration extraction method < aqueous extract by decoction extraction method < aqueous extract by sequential cold percolation extraction method < ethyl acetate extract by sequential cold percolation extraction method < toluene extract by sequential cold percolation extraction method.
Conclusion
The results of the present study showed that the sequential cold percolation extraction method was better than the decoction and maceration extraction methods maybe by concentrating active principles and by removing interferences to substances of the plant. Also, this could be due to the presence of an enormous amount of flavonoid and phenolic compounds, which are responsible for the immense antioxidant property. The study also revealed the possible antioxidant mechanism of the extracts such as hydrogen or electron-donating ability and direct free radical scavenging properties. The high scavenging property of S. cumini may be due to hydroxyl groups existing in the phenolic compounds that can scavenge the free radicals. The result of the present study would certainly help to ascertain the potency of the crude extract of leaves of S. cumini as potential source of natural antioxidants. However, further research is required to identify individual components forming antioxidative system and develop their application for pharmaceutical and food industries.
References
Amarowicz R, Estrella I, Hernandez T, Robredo S, Troszynska A, Kosinska A, Pegg RB (2010) Food Chem 121:705
An ZF (2000) Treatment of common diseases with drug liquors. Athletic, Beijing
Athukorala Y, Kim KN, Jeon YJ (2006) Food Chem Toxicol 44:1065
Banerjee A, Dasgupta N, De B (2005) Food Chem 90:727
Baravalia Y, Kaneria M, Vaghasiya Y, Parekh J, Chanda S (2009) Turk J Biol 33:159
Brighente IMC, Dias M, Verdi LG, Pizzolatti MG (2007) Pharm Biol 45:156
Cai YZ, Luo Q, Sun M, Corke H (2004) Life Sci 74:2157
Carrol KK, Kurowska EM, Guthirie N (1999) Int Patent WO 9916167
Chanda S, Dave R (2009) Afr J Microbiol Res 3:981
Chanda SV, Nagani KV (2010) Nature Sci 8:260
Chanda S, Dudhtra S, Kaneria M (2010) Food Funct 1:308
Chang C, Yang M, Wen H, Chern J (2002) J Food Drug Anal 10:178
Cragg MG, Newmann DJ (2001) Pharm Biol 39:8
Flora SJ (2007) Cell Mol Biol 53:1
Frankel EN, Kanner J, German JB, Parks E, Kinsella JE (1993) Lancet 341:454
Gholivand MB, Nasrabadi MR, Batooli H, Ebrahimabadi AH (2010) Food Chem Toxicol 48:24
Halliwell B, Gutteridge JMC (1981) FEBS Let 128:347
Halliwell B, Gutteridge JMC (1990) Methods Enzymol 186:1
Ito N, Fukushima S, Hagiwara A, Shibata M, Ogiso T (1983) J Nat Cancer Inst 70:343
Kalaivani T, Mathew L (2010) Food Chem Toxicol 48:298
Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S (2009) Indian J Pharm Sci 71:406
Ksouri R, Hanen Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, Bakrouf A, Magne C, Abdelly C (2009) Food Chem Toxicol 47:2083
Kunchandy E, Rao MNA (1990) Int J Pharm 58:237
Larbi A, Kempf J, Pawelec G (2007) Exp Gerontol 42:852
Lee KG, Mitchell AE, Shibamoto T (2000) J Agric Food Chem 48:4817
Li HB, Jiang Y, Wong CC, Cheng KW, Chen F (2007) Anal Bioanal Chem 388:483
Lima LA, Siani AC, Brito FA, Sampaio ALF, Henriques MGMO, Riehl CAS (2007) Quim Nova 30:860
Liu J, Wang C, Wang Z, Zhang C, Lu S, Liu J (2011) Food Chem 126:261
Maeda-Yamamoto M, Kawahara H, Tahara N, Tsuji K, Hara Y, Isemura M (1999) J Agric Food Chem 47:2350
Mantena RKR, Wijburg OLC, Vindurampulle C, Bennett-Wood VR, Walduck A, Drummond GR, Davies JK, Robins-Browne RM, Strugnell RA (2008) Cell Microbiol 10:1058
Martinez JA (2006) J Physiol Biochem 62:303
Mc Donald S, Prenzler PD, Antolovich M, Robards K (2001) Food Chem 73:73
McCune LM, Johns T (2002) J Ethnopharmacol 82:197
Meyer AS, Isaksen A (1995) Trends Food Sci Technol 6:300
Parekh J, Chanda S (2007) Braz J Microbiol 38:204
Rahman AU, Zaman K (1989) J Ethnopharmacol 26:1
Rakholiya K, Kaneria M, Chanda S (2011) J Med Plants Res 5:63
Ramarathnam N, Osawa T, Ochi H, Kawakishi S (1995) Trends Food Sci Technol 6:75
Rastogi RM, Mehrotra BN (1990) Compendium of Indian medicinal plants. Central Drug Research Institute, Lucknow, p 388
Robak J, Gryglewski RJ (1988) Biochem Pharmacol 37:837
Ruan PZ, Zhang LL, Lin YM (2008) Molecules 13:2545
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) J Agric Food Chem 40:945
Swerdlow RH (2007) Antioxid Redox Signal 9:1591
Teixeira CC, Pinto LP, Kessler FHP, Knijnik L, Pinto CP, Gastaldo GJ, Fuchs FD (1997) J Ethnopharmacol 56:209
Victor VM, Rocha M (2007) Curr Pharm Design 13:845
Warrier PK, Nambiar VPK, Ramankutty C (1996) Indian medicinal plants. Orient Longman Ltd, Hyderabad, p 225
Wiart C, Hannah A, Yassim M, Hamimah H, Sulaiman M (2004) J Ethnopharmacol 95:285
Williams RL, Elliots MS (1997) In: Shaihidi F (ed) Natural antioxidant: chemistry, health effects and applications. AOCS Press, Illinois, p 150
Yang D, Qiushuang W, Leqin K, Jianmei JB, Tiejin Y (2007) Asia Pac J Clin Nutr 16:158
Acknowledgments
The authors thank Prof. S.P. Singh, Head, Department of Biosciences, Saurashtra University, Rajkot, Gujarat, India for providing excellent research facilities. One of the authors, Mr. Mital Kaneria, is thankful to University Grants Commission, New Delhi, India for providing financial support as Junior Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chanda, S.V., Kaneria, M.J. Optimization of Conditions for the Extraction of Antioxidants from Leaves of Syzygium cumini L. Using Different Solvents. Food Anal. Methods 5, 332–338 (2012). https://doi.org/10.1007/s12161-011-9242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9242-0