Abstract
This paper presents a simple, fast, and sensitive method to determine zinc in samples of feces and fish feed by electrothermal atomic absorption spectrometry through the direct introduction of slurries of the samples into the spectrometer’s graphite tube. The procedure is based on the injection of 10 μL of an acidified aqueous solution containing 0.50% w/v of feces or feed and 0.50% v/v HNO3 into graphite tube. The limits of detection and quantification calculated for 20 readings of the blank of the standard slurries (0.50% w/v of feces or feed devoid of zinc) were 0.04 and 0.13 μg L−1 for the standard feces slurries and 0.05 and 0.17 μg L−1 for the standard feed slurries. The proposed method was applied in studies of digestibility of zinc in different fish feeds, and their results proved compatible with that obtained from samples mineralized by acid digestion using microwave oven.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc is a trace element whose role is essential in many biochemical processes. It is a component of hundreds of proteins involved in intermediary metabolism, hormone secretion pathway, and immune defense, and Zn enzymes participate in the synthesis and decomposition of proteins, fats, carbohydrates, and nucleic acids (McDowell 1992; Solomons 1992). The presence of this element in the cells is processed by protein carriers and in storage processes through metallothionein. A change in the concentration of this element in tissues can be observed in many pathological processes (Mertz 1987; McCall et al. 2000).
Because zinc participates in the metabolism of numerous enzymes, it is an essential element required for normal growth and is indispensable in animal diets (Christianson and Cox 1999; Yamaguchi 1998; Scarpa and Gatlin 1992). Thus, artificial diets for animals should contain the concentration of zinc required by the animal’s metabolism to maintain its health and high weight gain rates (Shearer et al. 1992). However, essential elements should also be in an available form for use by the organism (O’Dell 1984). Animal foods, such as fish food, usually contain an inorganic source of zinc to meet the species’ nutritional requirements, and these sources present significant zinc content. However, clinical signs of nutritional zinc deficiency can be observed when concentrations of zinc in the feed are inappropriate (Gatlin and Wilson 1984). Therefore, it is important to know the degree of zinc absorption and the bioavailability of this element in different food sources to allow for the formulation of efficient nutritional diets (McClain and Gatlin 1988).
However, determining the true zinc absorption in animals is a difficult task. The values of zinc bioavailability are usually expressed in relation to the response obtained with a standard reference material, which, in most cases, is zinc sulfate. A representative sample of the final blend with the other components of a feed, containing the exact zinc content in the food, is fundamental in determining the element. One of the main problems in the determination of zinc and other micronutrients in animal feed is excessive handling during the preparation of a sample for analysis. Therefore, there is an urgent need for the development of new methodologies for quantifying metal micronutrients in animal food. In this context, the determination of metal analytes in slurries by graphite furnace atomic absorption spectrometry (GFAAS) is a promising technique. This technique offers several advantages such as high sensitivity, extremely low detection limits, the use of small sample volumes, and the determination of a wide variety of trace elements. Moreover, since the atomizer can act as a chemical reactor, the possibility of analyzing solid samples offers advantages over conventional digestion procedures. Besides eliminating the stage of total prior decomposition of a sample, it reduces the sample preparation time, the loss of analytes through excessive handling or retention in insoluble products, potential contamination of the sample, and, in particular, minimizes the action of dangerous acids on the analyst (Miller-Ihli 1993; Bendicho and Loos-Vollebregt 1991; Liang et al. 1996).
In view of the above, this paper describes the development of a method to determine zinc in slurry samples of fish feed and feces by GFAAS. This method eliminates the mineralization step and allows the digestibility of zinc in fish food samples to be estimated.
Materials and Methods
Reagents, Standard Solutions, and Samples
High purity deionized water (18.2 MΩ cm−1) purified with an Elga Ionic system (PURELAB Option, USA), suprapure nitric acids (Merck), hydrogen peroxide (Merck), and Triton X-100 (Merck) were used throughout this work. The solution containing tungsten, which was employed to coat the inside of the graphite tube and to serve as a permanent modifier, was prepared by diluting a stock solution containing 1,000 mg L−1 of sodium tungstate (Merck) with ultrapure water. The Pd(II) solution, also employed as a chemical modifier, was similarly prepared, but palladium nitrate (Merck) was used instead.
Stock solutions of the analytes were prepared from reagents of spectroscopic purity (Johnson & Matthey, Royston, Hertfordshire, UK). The remaining solutions, including the concentrated acid solutions used for mineralizing the samples, were of analytical grade. All the bottles for storing samples and standard solutions, the glassware, and the atomic absorption spectrometer’s autosampler containers were immersed in 10% v/v nitric acid for 24 h, rinsed with ultrapure water, and dried before use.
The fish feces and feed samples were dried at 50 °C in an oven with forced air circulation for 48 h and then cryogenically ground. To this end, approximately 1.0 g of the sample, together with a magnetic bar, were put into a polycarbonate flask, which was then closed and immersed in liquid nitrogen. The impact between the sample and the magnetic bar, subjected to an oscillating magnetic field (20 impacts s−1), pulverized the sample. The sample-grinding program consisted of an initial stage of 2 min of prefreezing, 1 min of pulverization, another 1 min of freezing, followed by a second stage comprising two pulverization and freezing cycles, making a total time of 8 min. This procedure yielded particles with a granulometry of less than 60 μm (Rosa et al. 2002).
A portion of the samples was also mineralized in a microwave oven, as follows. Portions of 50 mg of cryogenically ground samples were transferred directly to the Teflon flasks of the microwave oven, and 2.5 mL of suprapure nitric acid 14 mol L−1 plus 0.50 mL of hydrogen peroxide 30% w/w were added. The heating program employed was the one described in the oven’s user manual.
Obtainment of the Biological Material Used for Standard Slurries’ Preparation
A feed devoid of calcium, iron, cobalt, cooper, magnesium, manganese, selenium, and zinc was formulated with dehydrated starch, albumin, and a premix containing potassium oxide. A group of Nile Tilapia Oreochromis niloticus juveniles was fed with this feed in a separate aquarium and their excrements were collected. After that, they were treated as previously described, except that they were exhaustingly washed with suprapure nitric acid 0.10 mol L−1, ultrapure water, and then dried.
Preparation of Slurry Samples
After cryogenic grinding, 20 mg of samples of biological material (fish feed or feces) were diluted 50 times with dried starch. Five milligrams of diluted samples were then transferred directly to the flasks of the spectrometer’s autosampler, to which were added 5 μL of suprapure nitric acid 14 mol L−1, 50 μL of Triton X-100 at 1% v/v, 100 μL of 1,000 mg L−1 Pd(II) solution, and 845 μL of ultrapure water. The slurry samples of biological material were then sonicated for 40 s directly in the autosampler’s flasks. The same procedure was carried out for preparation of the slurry corn bran (certified feed—RM 8433—National Institute of Standards and Technology) used to assess the accuracy of the proposed method, except that the solution was diluted 100 times.
Apparatus
A Provecto Analitica model DGT 100 plus microwave oven (Campinas, SP, Brazil) was used to mineralize the samples whenever necessary. Zinc determinations were carried out in an atomic absorption spectrometer Shimadzu model AA-6800, equipped with a self-reversal background correction system (SR) and an ASC-6100 autosampler furnished with longitudinally heated atomizers with integrated platform. A Shimadzu hollow cathode zinc lamp operated with a 10-mA current was also used. All measurements of Zn were made at 213.9 nm and based on integrated absorbance (or peak height) by using a spectral resolution of 0.5 nm. Argon was used as inert gas at a constant flow of 1 L min−1 throughout the heating program, except during the atomization step, when the gas flow was interrupted. The absorbance signals were measured in the peak area.
The samples were cryogenically ground in a cryogenic Spex 6750 Freezer Mill. The fish feces and feed slurries were shaken in a unique ultrasonic cell disruptor.
Preparation of the Graphite Tube Coated Internally with Tungsten
The inner walls of the pyrolytic graphite tubes with integrated platform used for determining zinc were coated with 500 μg W by injecting aliquots of 25 μL of a solution containing 1,000 mg L−1 of the sodium tungstate modifier into the atomizer, which was then subjected to the heating program described in Table 1. This procedure was repeated 20 times. When heated to 500 °C, the W(VI) deposited on the graphite tube’s inner wall forms a layer of tungsten carbide that acts as a chemical modifier (Fischer and Rademeyer 1998). In this case, the mass of tungsten deposited was 500 μg. This treatment enabled us to use the graphite tubes for up to 572 firings.
Preparation of the Standard Slurries
Analytical curves were prepared using fish feed and feces slurries containing 0.20, 0.40, 0.60, 0.80, and 1.00 μg L−1 of zinc with the absorbance readings done by GFAAS. These standard slurries were prepared under the same conditions as those applied in the preparation of the feed and feces sample slurries, but using 5 mg of standard samples of biological material devoid of zinc. To prepare the analytical curves, volumes of 4, 8, 12, 16, and 20 μL of standard solutions containing 50 μg L−1 of zinc were transferred to the spectrometer’s autosampler flasks before the final volume was adjusted to 1,000 μL with ultrapure water, so that the zinc concentrations in the standard slurries remained within a range of 0.20 to 1.00 μg L−1. The aqueous standard solutions containing 0.20 to 1.00 μg L−1 of zinc in 10% v/v suprapure HNO3 were also used in the construction of analytical curves in the determination of zinc in feces and feed samples mineralized by acid digestion in the microwave oven.
Analytical Procedures
After the slurry samples and/or standard slurries were sonicated directly in the autosampler’s containers, a volume of 10 μL of standard or sample was injected into the graphite tube (coated internally with tungsten carbide), using the autosampler’s micropipette. Each measurement was repeated five times. Table 2 describes the heating program of the graphite tube, which was optimized to determine zinc.
Results and Discussion
Optimization of the Instrumental Conditions
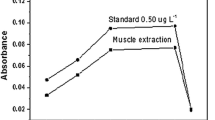
Obtaining exact and reproducible analytical results in the determination of metals by GFAAS using slurry samples depends on the optimization of the analyte’s pyrolysis and atomization temperatures. Therefore, pyrolysis and atomization curves were drawn to determine these parameters for zinc in standard fish feed and feces slurries containing 1.00 μg L−1 of zinc and 10 mg L−1 of Pd(II), using the graphite tube coated internally with tungsten carbide and the sample preparation conditions described under section “Preparation of Slurry Samples”. Figure 1 illustrates the influence of the pyrolysis and atomization temperatures on the zinc absorbance signal in the standard slurries of the biological materials. The pyrolysis temperature of 1,600 °C was selected because, as indicated in Fig. 1 (curves a and b), the zinc absorbance signals remained constant in the range of 1,400–1,600 °C, declining rapidly after reaching 1,600 °C. As for the atomization temperature (Fig. 1, curves c and d), the zinc absorbance signals were constant up to 2,400 °C for both feces slurries and feed slurries, so the atomization temperature of 2,400 °C was selected for all the remaining experiments. Figure 2 (graphs a and b) depicts the analyte absorbance (AA) and background (BG) absorbance signals for the standard slurries. Both graphs show a relatively low background absorbance, indicating the efficiency of Pd and tungsten carbide modifiers in the stage of pyrolysis. As the biological materials studied here displayed a magnesium content of about 0.12%, this element in the matrix may help to thermally stabilize zinc. According to the literature on some analytes, e.g., chromium and selenium, the presence of magnesium in the matrix favors the thermal stabilization of these elements (Rosa et al. 2002; Aleixo et al. 2000).
Pyrolysis and atomization temperature curves of the standard samples containing 1.00 μg L−1 of Zn. Pyrolysis curves (atomization temperature, 2,400 °C)—a feces standard slurry, b feed standard slurry; atomization curves (pyrolysis temperature, 1,600 °C)—c feces standard slurry, d feed standard slurry
Determination of the Optimal Ultrasonication Time of the Slurry Samples
Ultrasonic shaking prior to the analysis of slurries of solid materials provides good homogenization of the sample and, consequently, better reproducibility between measurements (Rosa et al. 2002). Thus, the ultrasonication time was evaluated in the interval of 10 to 60 s of agitation. Figure 3 depicts the influence of the ultrasonication time of samples on the zinc absorbance signals and the relative standard deviation (RSD) of each ultrasonication time. An analysis of this figure indicates that the absorbance signals remained constant starting from 30 s of sonication. A sonication time of 40 s was considered optimal, providing a good absorbance signal and a relatively low RSD (1.8%).
Obtainment of the Analytical Curves
Based on the parameters of pyrolysis and atomization temperature, in the profile of the optimized atomic absorption signal and using the calibration system by matrix matching procedure (Lima et al. 1998), analytical curves were plotted using standard fish feces and feed slurries containing zinc in concentrations of 0.20 to 1.00 μg L−1 (as described earlier under the item “Preparation of the Standard Slurries”). Figure 4 depicts the analytical curves and their respective straight-line equations. A comparison of the analytical curve prepared with fish feces and the one prepared with fish feed (Fig. 4) indicated that their slopes did not show significant differences (k = 0.3322 for feces curve and k = 0.3216 for feed curve). Both analytical curves showed absorbance values approximately 2–5% lower than the values of the analytical curve obtained from the standard solutions prepared in the range of 0.20 to 1.00 μg L−1 of zinc in 10% v/v suprapure HNO3 (straight line equation: \(A = {\text{0}}{\text{.0321}} + {\text{0}}{\text{.1656}}k\)). However, the slopes of these straight lines were about twofold greater for the standard slurries, attesting to the efficiency of the pyrolysis and atomization temperature stage of the heating schedule applied here. An accumulation of carbonaceous residues inside the graphite tube, causing partial obstruction of the radiation from the hollow cathode lamp, can impair absorbance measurements (Aleixo et al. 2000). Nevertheless, the low BG signals obtained in the zinc optimization stage of the proposed procedure indicated that the carbon residue left by the pyrolysis stage did not impair the absorbance measurements. The characteristic calculated masses were 2.70 and 2.74 pg for the standard feces and feed slurries, respectively. The limit of detection (LOD) and of quantification (LOQ) were calculated based on the standard deviation of 20 readings obtained for the blanks of the standard slurries and the slopes of the analytical curves (LOD = 3σ/slope and LOQ = 10σ/slope). LOD was 0.04 and LOQ was 0.13 μg L−1 of zinc in the standard feces slurry and 0.05 and 0.17 μg L−1 of zinc in the standard feed slurry, respectively (Rosa et al. 2002; Lima et al. 1999; Minami et al. 1997). A comparison was made of the LOD obtained by the proposed method against that obtained by GFAAS using aqueous standard solutions (0.03 μg L−1 of zinc), whenever they showed equivalent values. The results of this comparison demonstrate that the proposed method offers an acceptable level of precision. The lifetime of the graphite tube was equivalent to 572 firings. Considering the complexity of the biological matrices, the tube’s service life using the proposed method is acceptable when compared with other methods described in the literature (Rosa et al. 2002; Aleixo et al. 2000; Lima et al. 1999; Minami et al. 1997). After optimization, the accuracy of the proposed method for determining zinc was investigated in slurries of four feed samples and a standard reference material of corn bran, RM 8433—National Institute of Standards and Technology—which was used in the fish feed. Zinc determinations were also carried out in the feces of fish fed with four samples of feed. The results obtained are shown in Table 3. The accuracy of the results was checked by mineralizing the samples in a microwave oven, which indicated that they were not statistically different at a 95% level of confidence (paired t test).
Application of the Proposed Method
After the procedures of optimization and the LOD and LOQ determination, the applicability of the newly developed method was tested by determining zinc in four feed samples containing different zinc supplements used in the diet of Nile tilapia juveniles and in samples of feces from these fish. Then, based on the values of the percentage of Cr2O3 and of zinc determinations of the feeds, the coefficient of digestibility of this micronutrient was calculated, using Eq. 1 (Sá et al. 2004).
where Da is the apparent digestibility, % Cr2O3 r is the percentage of chromic oxide in the feed, % Cr2O3f is the percentage of chromic oxide in the feces, % Znr is the percentage of zinc in the feed, and % Znf is the percentage of zinc in the feces.
The values of the zinc digestibility coefficients of the four types of feed used in the diet of Nile tilapia juveniles were compared, based on the zinc determination by the proposed method and by GFAAS after mineralization of the feed samples in a microwave oven, demonstrating that these values were equivalent. The GFAAS method is normally used in fish nutrition mineral bioavailability studies (Furuya et al. 2001; Pezzato et al. 2002; Sá et al. 2005); hence, our results attest to the applicability of the proposed method in such studies (Table 4).
Thus, the proposed method for quantifying zinc using fish feed and feces samples in the form of suspensions to estimate the digestibility of this micronutrient in feeds used in fish nutrition yielded results equivalent to those obtained with the GFAAS quantification method, whose initial step involves the mineralization of samples in a microwave oven. The main advantage of the proposed method is that it does not generate toxic residues, which can be harmful to the analyst’s health and contaminate the environment. Moreover, since this new method does not require mineralizing the samples, it considerably reduces the time spent on analytical determinations in fish nutrition analysis.
References
Aleixo PC, Nóbrega JA, Junior DS, Muller RCS (2000) Quim Nova 23:310
Bendicho C, Loos-Vollebregt MTC (1991) J Anal At Spectrom 6:353
Christianson DW, Cox JD (1999) Annu Rev Biochem 68:33
Fischer JL, Rademeyer CJ (1998) Spectrochim Acta B 53:549
Furuya WM, Pezzato LE, Pezzato AC, Barros MM, Miranda EC (2001) Braz J Anim Sci 30:1143
Gatlin DMIII, Wilson RP (1984) Aquaculture 41:31
Liang Y, Li M, Rao Z (1996) Anal Sci 12:629
Lima EC, Krug FJ, Jackson KW (1998) Spectrochim Acta Part B 53:1791
Lima EC, Krug FJ, Ferreira AT, Junior FB (1999) J Anal At Spectrom 14:269
McCall KA, Huang C, Fierke CA (2000) J Nutr 130:1437
McClain WR, Gatlin DMIII (1988) J World Aquac Soc 19:103
McDowell LR (1992) Minerals in animal and human nutrition. Academic, London, p 524
Mertz W (1987) Trace elements in human and animal nutrition. Academic, San Diego, pp 1–480
Miller-Ihli NJ (1993) J Anal Chem 345:482
Minami H, Inoue Y, Sakata K, Atsuya I (1997) Anal Sci 13:397
O’Dell BL (1984) Nutr Rev 42:301
Pezzato LE, Miranda EC, Barros MM, Pinto LGQ, Furuya WM, Pezzato AC (2002) Braz J Anim Sci 31:1595
Rosa CR, Moraes M, Neto JAG, Nóbrega JA, Nogueira ARA (2002) Food Chem 79:517
Sá MVC, Pezzato LE, Lima MMBF, Padilha PM (2004) Aquaculture 238:385
Sá MVC, Pezzato LE, Barros MM, Padilha PM (2005) Aquac Nutr 11:273
Scarpa J, Gatlin DM III (1992) Aquaculture 106:311 doi:10.1016/0044-8486(92)90263-K
Shearer KD, Maage A, Opstvedt J, Mundheim H (1992) Aquaculture 106:345 doi:10.1016/0044-8486(92)90266-N
Solomons NW (1992) Absorption and malabsorption of mineral nutrients. Liss, New York, p 125
Yamaguchi M (1998) J Trace Elem Exp Med 11:119 doi:10.1002/(SICI)1520-670X(1998)11:2/3<119::AID-JTRA5>3.0.CO;2-3
Acknowledgments
The authors gratefully acknowledge the financial support of FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo (Process 03/13362-6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saleh, M.A.D., de Cássio Ferreira Neves, R., Silva, F.A. et al. GFAAS Determination of Zinc in Fish Feed and Feces Using Slurry Sampling. Food Anal. Methods 2, 162–168 (2009). https://doi.org/10.1007/s12161-008-9053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-008-9053-0