Abstract
Background
African American men experience increases in smoking during the young adult transition. Exposure to childhood adversity, a risk factor which disproportionately affects African American men, has been identified as a robust precursor to health risk behavior in general and cigarette smoking in particular. The intermediate mechanisms that transmit the influence of early adversity to smoking behavior are not well understood.
Purpose
We tested a model of the escalation of smoking behaviors among young adult African American men, investigating sleep disturbance and delayed reward discounting as intermediate factors linking adverse childhood experiences with smoking.
Methods
Hypotheses were tested with three waves of data (M age-T1 = 20.34, M age-T2 = 21.92, M age-T3 = 23.02) from 505 African American men living in rural counties in South Georgia. Men provided self-report data on their adverse childhood experiences, sleep problems, and smoking behavior using audio-assisted computer self-interviews. Men also completed a computer-based delayed reward discounting task.
Results
Structural equation modeling analyses supported our hypotheses: Adverse childhood experiences predicted poor sleep adequacy, which forecast increases in delayed reward discounting; discounting, in turn, predicted increased smoking. Significant indirect pathways were detected linking adversity to discounting via sleep adequacy and linking sleep adequacy to smoking via discounting.
Conclusions
Prevention and intervention researchers can draw on these findings to develop programs that focus on sleep adequacy to reduce smoking in African American men exposed to childhood adversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tobacco use is significantly associated with heart disease, cancer, and stroke, which are the three leading causes of death among African American men [1,2,3]. Patterns of smoking initiation and escalation among African American men indicate low levels of smoking in adolescence [4] with rapid increases in both initiation and escalation during young adulthood. By age 30, the prevalence of smoking among African American men exceeds the national average [5]. Among African American men from stressful, low-socioeconomic status (SES) communities, prevalence rates may exceed twice the national average, ranging from 41 to 60% of adult men [6]. These data underscore the importance of understanding those factors associated with the initiation and escalation of smoking among young African American men, particularly those from low-SES environments.
Adverse childhood experiences refer to events during childhood that exceeds a child or youth’s coping resources. Examples include physical, sexual, emotional, and verbal abuse; neglect; and household dysfunctions, such as witnessing inter-parental violence. Such experiences are linked to addictive behaviors in general and cigarette smoking in particular [7, 8]. Although the association between childhood adversity and smoking tobacco is robust [8, 9], the intermediate processes linking adversity to smoking are not well understood. The lack of this information is particularly problematic given data indicating that exposure to childhood adversity is more prevalent among male African Americans than members of other ethnic/gender groups [10].
Exposure to adverse childhood experiences triggers a multitude of somatic and neurocognitive alterations in the developing child that may be associated with risk for downstream smoking [11, 12]. Drawing on emerging data linking adverse childhood experiences to sleep problems [13, 14] and alterations in decision-making processes associated with smoking [15], we tested an indirect effect model of the pathways linking adversity to smoking. We hypothesized that sleep-related problems would represent a novel mechanism informing the etiological pathways linking adversity to risky decision-making, a proximal vulnerability factor for smoking. Sleep disturbances are common among US adults and include problems associated with falling asleep, sleep quality, and obtaining sufficient amounts of sleep [16]. Accumulating evidence links childhood adversity to sleep disturbance [17]. A positive graded relationship between exposure to childhood adversities and sleep problems has been documented with both self-report and objective sleep measures [17, 18]. At present, the mechanisms through which adversity affects adult sleep are unclear. Recent research implicates disruptions in stress regulatory systems associated with HPA axis activity [14]. Other research suggests that sleep disturbances may simply represent instability in the social environment, as individuals who grew up with adversity continue to live in unstable and unsupportive environments that undermine sleep hygiene [14]. Of particular interest, sleep problems have been implicated in a broad range of health problems and health risk behaviors, including tobacco use. Smokers report greater difficulties with falling asleep, waking up, and daytime sleepiness than do nonsmokers, and adolescents with sleep problems are more likely to initiate tobacco use than are those with few sleep problems [19].
The potential for sleep problems to operate as an intervening process linking childhood adversity to smoking behavior has yet to be investigated. We hypothesize that sleep problems affect smoking frequency among men indirectly, via influences on delayed reward discounting (DRD). DRD indexes a person’s preference for proximate but smaller rewards compared with delayed but larger rewards [20]. Conceptually, DRD is similar to difficulty in delaying gratification. The focus of DRD, however, is on valuing immediate rewards versus future ones rather than one’s ability to wait for a reward. Studies show that individuals high in DRD exhibit a preference for the immediate rewards of smoking while minimizing its future health consequences [21]. Youth high in DRD are more likely than those low in DRD to begin smoking and to experience relatively rapid smoking escalation [22].
Emerging theoretical perspectives [12] and empirical evidence [23, 24] suggest that DRD is altered by both a history of childhood adversity and sleep problems. Adverse rearing environments may have direct effects on the development of decision-making. In a harsh and unreliable rearing context, developing children may experience little or no reinforcement for delaying gratification or hoping for larger rewards in the future [25]. Over time, the developing child learns to prefer immediate rewards, resulting in a tendency toward impulsive decision-making. Harsh environments may also affect cognitive functioning indirectly, via influences on sleep. Accumulating evidence suggests that chronically inadequate sleep takes a toll on adolescents’ and young adults’ neurocognitive functioning and attendant decision-making processes [26, 27]. Chronically inadequate sleep is linked to decision-making processes associated with DRD as well as to substance abuse in adolescence and young adulthood [28, 29].
The potential for chronic sleep deprivation and other aspects of sleep pathology (poor sleep quality, difficulty falling or staying asleep) to influence the associations among adverse childhood experiences, DRD, and tobacco use has yet to be investigated empirically. Using a prospective design, we tested an indirect effect model in which childhood adversity was hypothesized to be associated positively with sleep problems, resulting in elevated DRD, a proximal predictor of smoking escalation. Hypotheses were tested with 505 African American men from resource-poor rural environments who participated in a three-wave prospective study of health risk behavior. Men’s mean ages were 20 at baseline and 23 at the second follow-up assessment, allowing for an examination of smoking-related antecedents during a period when many African American men begin smoking and develop nicotine dependence.
Methods
Participants
Participants included 505 African American men who resided in one of 11 rural counties in South Georgia, an area representative of a geographic concentration of rural poverty across the southern coastal plain [30]. Men’s mean age was 20.34 years (SD = 1.21; range 19 to 22) at the baseline interview (time 1; T1). Participants were recruited using respondent-driven sampling (RDS), which combines a prescribed chain-referral recruitment method with a mathematical model that allows for post-stratification sample weighting. Community liaisons recruited 45 initial seed participants from targeted counties to complete a baseline survey. Each participant was then asked to identify three other men in his community from his personal network who met the criteria for inclusion in the study (African American, age 19–22, and living in the targeted area). Project staff contacted the referred potential participants, and the referring participant received $25 per person who completed the survey. After completing the survey, each referred participant, in turn, was asked to refer three men in his network. The RDS protocols are designed to attenuate the influence of biases common in chain-referral samples and to improve the approximation of a random sample of the target population [31, 32]. Analyses of network data related to substance use and other risky behavior at T1 [33, 34] indicated that the sample evinced negligible levels of common biases observed in chain-referral samples arising from the characteristics of the initial seed participants, the recruitment efficacy of individual participants, and differences in the sizes of participants’ networks.
Data Collection Procedures and Retention
African American research staff visited participants in the participants’ homes or at convenient community locations, where participants completed an audio computer-assisted self-interview on a laptop computer. This procedure allowed participants to navigate the survey privately with the help of voice and video enhancements, eliminating literacy concerns. Approximately 1.5 years later, when men’s mean age was 21.92 years (SD = 1.35), a follow-up data collection visit (time 2; T2) was conducted in the same manner. A third visit (time 3; T3) took place approximately 1 year after T2; men’s mean age at T3 was 23.02 (SD = 1.24). Of the 505 men who participated at T1, 423 (83.8%) completed the T2 survey and 408 (80.8%) completed the T3 survey. Retention status was not associated with any study variables. Participants received $100 at each time point for completing the surveys. Participants provided written informed consent at baseline, and all study protocols were approved by the Institutional Review Board of the university at which the study was conducted.
Measures
Adverse Childhood Experiences
At T1, men reported the presence or absence of 16 types of adverse childhood experiences during their first 16 years of life using the Adverse Childhood Experiences (ACE) Questionnaire [35]. Adversities included experiencing physical abuse, neglect, or sexual abuse and witnessing violence directed toward one’s caregiver. Scores ranged from 0 to 16, with a mean of 2.82 (SD = 2.97); Cronbach’s α = 0.76.
Sleep
At T1, participants completed the Medical Outcomes Study (MOS) sleep scale [36], a 12-item survey assessing six sleep dimensions: initiation (time to fall asleep), quantity (hours of sleep each night), maintenance, respiratory problems, perceived adequacy, and somnolence. Participants responded on a 5-item Likert scale ranging from 0 (“none of the time”) to 4 (“all of the time”) based on their sleep during the past 4 weeks. Past research documents the reliability of the subscales and their convergent and discriminant validity [37, 38], including associations with observational assessments of sleep pathology [39]. Little research, however, has examined sleep specifically with young African American men; preliminary analyses indicated that the extant subscales lacked reliability in our sample (α = 0.43). We thus re-evaluated the factor structure of the measure using an exploratory factor analysis (EFA; oblique rotation; factor selection based on eigenvalue >1; and assessment of a parallel process scree plot). Details are available from the first author. Analyses revealed three distinct factors: poor sleep quality (four items: “sleep not quiet,” “awake short of breath or with a headache,” “feel drowsy or sleepy during the day,” and “have trouble staying awake during the day”), sleep inadequacy (three items: “get the amount of sleep needed,” “hours slept per night,” and “get the amount of sleep needed,” reverse coded), and difficulty falling asleep (three items: “trouble falling asleep,” “time taken to fall asleep,” and “awake during sleep and have trouble falling asleep again”). To ensure the internal consistency of the three factors in SEM, a confirmatory factor analysis was conducted (see Table 2). In the subsequent SEM modeling, we used the latent factors that the EFA had yielded and named them poor sleep quality, sleep inadequacy, and difficulty falling asleep.
Delayed Reward Discounting
DRD was assessed with the Monetary Choice Questionnaire (MCQ) at T1 and T2 [40]. The MCQ consists of 27 items that pair a small-immediate reward and a larger-delayed reward (e.g., “Would you rather have $54 today or $55 in 117 days?”). Participants were instructed to choose the rewards they preferred. The MCQ provides estimates of an individual’s temporal discounting of rewards at three magnitudes (small, $25–35; medium, $50–60; large, $75–85). To enhance the assessment’s validity, participants received one randomly selected actual reward from the items. The MCQ predicts addictive behavior with effect sizes similar to those that extended decision-making tasks yield [41]. Per Kirby et al., we calculated hyperbolic discounting functions (k) for each of the three magnitudes (small, medium, and large rewards), with higher k values representing elevated hyperbolic discounting functions indicative of impulsive decision-making.
Smoking
Men reported their cigarette smoking at T1, T2, and T3 in response to the question, “In the past three months, how much did you smoke cigarettes?” Possible responses were 0 (none at all), 1 (less than one cigarette a day), 2 (1 to 5 cigarettes a day), 3 (about a half a pack a day), 4 (about a pack a day), 5 (about 1 and a half packs a day), 6 (about 2 packs a day), and 7 (more than two packs a day). Test-retest reliability of self-reported tobacco use is high compared with other health risk behaviors (e.g., alphas exceeding 0.80 for cigarettes consumed in the past 30 days versus 0.62 for seat belt use) [42], and single-item self-reports are associated with serum cotinine levels among young adults (0.46–0.64, p < .0001) [43].
Plan of Analysis
Hypotheses were tested using structural equation modeling (SEM) with maximum likelihood estimation as implemented in Mplus Version 7.4 [44]. Missing data (average missing rate: 7.0%) were imputed using Bayesian analysis [44]. A multiple imputation approach was used with ten imputation data sets generated by Mplus. Smoking at T1, T2, and T3 were square root transformed to meet normality assumptions. We specified a model with ACE predicting three latent sleep factors (poor sleep quality, sleep inadequacy, and difficulty falling asleep) at T1. Sleep constructs, in turn, were modeled as predictors of DRD at T2 with DRD levels at T1 controlled. DRD at T2 was specified as a predictor of cigarette smoking at T3 with smoking levels at T2 controlled. Because smoking is a potential contributor to DRD, we included smoking at T1 as a predictor of DRD. Indirect effects were assessed with the product-of-coefficients (α*β) approach [45], and confidence intervals were obtained using the RMediation package [46]. RMediation produces confidence intervals (CIs) using methods based on the distribution of product, Monte Carlo simulations, and an asymptotic normal distribution. Statistical fit criteria that Hu and Bentler suggested [47] were used to assess model fit.
Results
Preliminary Analyses
Table 1 presents descriptive statistics and bivariate correlations among study variables. Smoking rates were similar at all three time points. Approximately 60% of the men reported that they did not smoke (T1, 59.6%; T2, 61.7%; T3, 61.9%); around 14 to 16% smoked one to five cigarettes per day (T1, 15.8%; T2, 14.4%, T3, 13.7%), and approximately 6 to 8% reported smoking one to two packs of cigarettes per day (T1, 7.5%; T2, 7.3%, T3, 6.2%). Men reported a mean of 2.8 (SD = 2.97) adverse childhood experiences, more than twice the number found in representative samples of adults in Georgia [48]. The most common ACE reported was parental divorce (51.9%), followed by verbal abuse (28.5%) and physical abuse (24.2%).
Prior to testing our indirect effect hypotheses, we examined the measurement model of sleep constructs with a confirmatory factor analysis (Table 2). The model fit the data well: χ 2(29) = 32.752, p = .288; RMSEA = 0.016; SRMR = 0.031; CFI = 0.99; TLI = 0.99. All parameters loaded on their respective factors significantly (p < .001), in the expected direction, and with factor loadings exceeding 0.30. The covariance between poor sleep quality and difficulty falling asleep was β = 0.575, p < .001, 95% CI [0.200, 0.358]; between poor sleep quality and sleep inadequacy, β = 0.508, p < .001, 95% CI [0.066, 0.165]; and between difficulty falling asleep and poor sleep quality, β = 0.440, p < .001, 95% CI [0.056, 0.153].
Primary Analyses
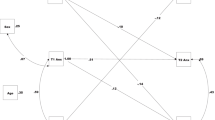
Table 3 and Fig. 1 present the analysis of the indirect effect model. The model fit the data well: χ 2(88) = 144.737, p < .001; CFI = 0.938; TLI = 0.919; RMSEA = 0.036, SRMR = 0.048. Adverse childhood experiences were associated positively with poor sleep quality (β = 0.148, p < .01, 95% CI [0.298, 1.336]), difficulty falling asleep (β = 0.182, p < .01, 95% CI [0.506, 2.055]), and sleep inadequacy (β = 0.120, p < .05, 95% CI [0.001, 0.009]). Sleep inadequacy was positively associated with DRD at T2 (β = 0.224, p < .01, 95% CI [0.337, 2.333]), after adjusting for the influence of baseline levels of DRD and smoking. Poor sleep quality (β = −0.112, p = .171, 95% CI [−0.012, 0.002]) and difficulty falling asleep (β = −0.121, p = 0.226, 95% CI [−0.011, 0.003]), however, were not significantly associated with DRD at T2. DRD at T2 predicted smoking at T3 (β = 0.153, p < .01, 95% CI [0.043, 0.263]) net of smoking levels at T2. Indirect effect tests indicated a significant indirect effect of adverse childhood experiences on DRD through sleep inadequacy (α*β = 0.027, p < .05, 95% CI [0.001, 0.015]) and a significant indirect effect of sleep inadequacy on smoking through DRD (α*β = 0.031, p < 0.05, 95% CI [0.027, 0.458]).
Discussion
Adverse childhood experiences constitute a robust predictor of a wide range of health risk behaviors, including cigarette smoking [8, 11]. Recent advances in developmental science suggest that exposure to adverse childhood experiences can potentiate somatic and neurocognitive alterations that are linked to health risk behavior [49], including smoking [50]. In the present study, we advanced and tested a longitudinal model of the intervening mechanisms that underlie the associations between adverse childhood experiences and cigarette smoking. Using a longitudinal sample of African American men, we found that adverse childhood experiences predicted multiple sleep problems; sleep inadequacy in particular affected smoking via increases in DRD.
Our findings suggest that sleep problems in general, and chronic problems with obtaining sufficient amounts of sleep in particular, represent a potential mechanism for understanding the negative health effects of childhood adversity. To our knowledge, this is the first study to investigate sleep problems as a mechanism in the path linking exposure to adverse childhood experiences to smoking. Examinations of the consequences of childhood adversity for addictive behaviors are particularly salient given evidence of disproportionate rates of adverse childhood experiences among African American men [51]. In the present study, exposure to childhood adversity was associated with men’s self-reported difficulty falling asleep, poor quality of sleep, and sleep inadequacy. This finding is consistent with a growing body of research linking a range of adverse experiences in childhood and adolescence to adult sleep problems [14, 52]. The mechanisms linking adversity to sleep problems are at present unclear. Adversity may initiate patterns of sleep difficulty that begin in childhood and continue into adulthood, as well as compromising the function of stress-related neurobiological systems associated with sleep regulation [18].
Our findings suggest that insufficient amounts of sleep during a 4-week period contribute to young African American men’s smoking behavior. Recent clinical research on sleep deprivation suggests a direct link to cigarette smoking [19]. This research specifies insomnia as a trigger for smoking among those who smoke [26, 53]. The present study extends this literature by examining the indirect effect of sleep on smoking via decrements in neurocognitive functioning. Our findings are consistent with multiple studies indicating that acute and chronic sleep deprivation significantly affects reward pathways linked with addictive behaviors [27, 54, 55]. In our study, men who reported at baseline inadequate sleep during the past 4 weeks displayed increases in DRD, the tendency to discount the value of future rewards. Thus, sleep problems, rather than acting as an immediate trigger for smoking, may undermine decision-making processes over time, leading to chronic vulnerability to poor decision-making in general and increases in smoking in particular.
Findings associated with the effects of insufficient sleep on smoking via DRD are consistent with an emerging literature that documents the neurocognitive consequences of inadequate sleep [56]. Sleep deprivation is a reliable predictor of executive functioning, including attentional processes, inhibitory control of behavior, and emotional reactivity associated with impulsivity [29, 57, 58]. Individuals who are deprived of sleep show amplified reactivity in brain reward networks, such as the amygdala, that are linked to biased appraisals of positive and negative emotional experiences [55, 59]. Studies indicate that these influences accumulate over time among individuals with chronic sleep problems [29]. DRD is considered a measure of decision-making that indexes reward system functioning that both state and trait characteristics influence [60]. Taken together, emerging research and the present study suggest that insufficient sleep may disrupt the balance between emotional and cognitive control systems, undermining the decision-making processes that help one to avoid health-compromising behaviors such as cigarette smoking [27].
Two aspects of sleep pathology were not related to DRD: difficulty falling asleep and poor-quality sleep. The majority of research examining neurocognitive outcomes focuses on sleep deprivation, a construct that is most similar to the sleep inadequacy scale in the present study. Although less well studied, other aspects of sleep-related problems demonstrate less consistent links with neurocognitive functioning in general, although research documents associations with depression (trouble falling asleep) and anxiety (restless, disturbed sleep) [61]. In these cases, however, it is unclear if sleep disturbance is a result or a cause of dysfunction. Additional research is needed to determine whether particular aspects of sleep pathology are more salient for understanding alterations in cognitive versus emotional functioning.
The study’s findings suggest a novel target for intervention programs designed to prevent smoking among African American men, particularly those exposed to childhood adversity. Research shows that adults exposed to childhood adversity exhibit heightened difficulty with smoking cessation, even when they contract smoking-related chronic diseases [62]. Smoking cessation is often accompanied by exacerbation of sleep problems. Addressing the sleep needs of young African American men prior to smoking initiation and in the context of cessation treatment may be a critical element of a comprehensive strategy [63]. Although data on sleep informed smoking prevention interventions are scarce, improved sleep has been shown experimentally to enhance decision-making processes associated with smoking initiation and escalation [54].
The study methods include several noteworthy strengths. Prospective studies of low-SES African American men that capture diverse environmental and personal risk factors for addictive behavior are rare. The use of a prospective design permitted examination of increases in both DRD and smoking behavior over time. Assessments occurred during a critical period when epidemiological data indicate that many African American men experience rapid escalation of smoking behavior; thus, this study captures changes during an important developmental transition. The study is also limited in several respects. Self-report assessments are subject to recall and desirability biases. This is mitigated somewhat by the use of widely validated measures that evince strong associations with objective assessments of study constructs. Future studies that incorporate prospective data on childhood adversity, as well as additional facets of impulsivity [64] and smoking biomarkers (e.g., expired carbon monoxide, cotinine, nicotine metabolic ratio), are needed to validate the present findings. The ACE measure also does not capture parental separations among never married, cohabitating parents which occur with greater frequency among African American samples than other racial groups. In addition, although the present study sheds light on an underserved and high-risk rural sample, its generalizability is limited. The findings may not characterize African American men in nonrural environments. Finally, when measuring DRD, a limitation of the MCQ is that it does not account for perceived incentive value, which may also be influenced by income or SES. Thus, it is possible that participants’ responses on the DRD were influenced by impulsivity, perceived incentive value, or both. Nevertheless, this longitudinal investigation illuminates the intervening factors linking childhood adversity and smoking behavior in a vulnerable and understudied population.
References
Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation. 2010, 121:e46-e215.
Health UDo, Services H: The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2004, 62.
Romero CX, Romero TE, Shlay JC, Ogden LG, Dabelea D: Changing trends in the prevalence and disparities of obesity and other cardiovascular disease risk factors in three racial/ethnic groups of USA adults. Advances in preventive medicine. 2012, 2012.
Tucker JS, Ellickson PL, Klein DJ: Predictors of the transition to regular smoking during adolescence and young adulthood. Journal of Adolescent Health. 2003, 32:314–324.
Jamal A, Homa DM, O’Connor E, et al.: Current cigarette smoking among adults—United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015, 64:1233–1240.
Delva J, Tellez M, Finlayson TL, et al.: Cigarette smoking among low-income African Americans: A serious public health problem. American journal of preventive medicine. 2005, 29:218–220.
Shonkoff JP, Garner AS, Siegel BS, et al.: The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012, 129:e232-e246.
Anda RF, Croft JB, Felitti VJ, et al. Adverse childhood experiences and smoking during adolescence and adulthood. Jama. 1999, 282:1652–1658.
Dube SR, Felitti VJ, Dong M, et al.: Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics. 2003, 111:564–572.
Umberson D, Thomeer MB, Williams K, Thomas PA, Liu H: Childhood adversity and men’s relationships in adulthood: Life course processes and racial disadvantage. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015:gbv091.
Sinha R, Jastreboff AM: Stress as a common risk factor for obesity and addiction. Biological psychiatry. 2013, 73:827–835.
Lovallo WR: Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. International journal of psychophysiology. 2013, 90:8–16.
Chapman DP, Liu Y, Presley-Cantrell LR, et al. Adverse childhood experiences and frequent insufficient sleep in 5 US States, 2009: A retrospective cohort study. BMC public health. 2013, 13:1.
Chapman DP, Wheaton AG, Anda RF, et al.: Adverse childhood experiences and sleep disturbances in adults. Sleep medicine. 2011, 12:773–779.
Koob G, Kreek MJ: Stress, dysregulation of drug reward pathways, and the transition to drug dependence. American Journal of Psychiatry. 2007, 164:1149–1159.
Gradisar M, Gardner G, Dohnt H: Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep medicine. 2011, 12:110–118.
Bader K, Schaefer V, Schenkel M, Nissen L, Schwander J: Adverse childhood experiences associated with sleep in primary insomnia. Journal of sleep research. 2007, 16:285–296.
Koskenvuo K, Hublin C, Partinen M, Paunio T, Koskenvuo M: Childhood adversities and quality of sleep in adulthood: A population-based study of 26,000 Finns. Sleep medicine. 2010, 11:17–22.
Hamidovic A, de Wit H: Sleep deprivation increases cigarette smoking. Pharmacology Biochemistry and Behavior. 2009, 93:263–269.
Bickel WK: Discounting of delayed rewards as an endophenotype. Biol. Psychiatry. 2015, 77:846–847.
Ohmura Y, Takahashi T, Kitamura N: Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Behavioral Economics of Preferences, Choices, and Happiness: Springer, 2016, 179–196.
Reynolds B, Karraker K, Horn K, Richards JB: Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behavioural Processes. 2003, 64:333–344.
Lovallo WR, Farag NH, Sorocco KH, et al. Early life adversity contributes to impaired cognition and impulsive behavior: Studies from the Oklahoma Family Health Patterns Project. Alcoholism: clinical and experimental research. 2013, 37:616–623.
VanderBroek L, Acker J, Palmer AA, de Wit H, MacKillop J: Interrelationships among parental family history of substance misuse, delay discounting, and personal substance use. Psychopharmacology. 2016, 233:39–48.
Ellis BJ, Oldehinkel AJ, Nederhof E: The adaptive calibration model of stress responsivity: An empirical test in the Tracking Adolescents’ Individual Lives Survey study. Development and Psychopathology. 2016:1–21.
Johnson EO, Breslau N: Sleep problems and substance use in adolescence. Drug and alcohol dependence. 2001, 64:1–7.
Telzer EH, Fuligni AJ, Lieberman MD, Galván A: The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage. 2013, 71:275–283.
Acheson A, Richards JB, de Wit H: Effects of sleep deprivation on impulsive behaviors in men and women. Physiology & Behavior. 2007, 91:579–587.
Alhola P, Polo-Kantola P: Sleep deprivation: Impact on cognitive performance. Neuropsychiatric disease and treatment. 2007, 3:553.
Crockett LJ, Carlo G, Temmen C: Ethnic and racial minority youth in the rural United States: An overview. Rural ethnic minority youth and families in the United States: Springer, 2016, 1–12.
Heckathorn DD: Respondent-driven sampling: A new approach to the study of hidden populations. Social problems. 1997, 44:174–199.
Heckathorn DD: Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Social problems. 2002, 49:11–34.
Kogan SM, Cho J, Barton AW, et al.: The influence of community disadvantage and masculinity ideology on number of sexual partners: A prospective analysis of young adult, rural black men. The Journal of Sex Research. 2016:1–7.
Kogan SM, Cho J, Oshri A: The influence of childhood adversity on rural black men’s sexual risk behavior. Annals of behavioral medicine. 2016, 50:813–822.
Felitti V, Anda R: The adverse childhood experiences (ACE) study. Centers for Disease Control and Prevention. 1997.
Hays RD, Stewart A: Sleep measures. 1992.
Hays RD, Martin SA, Sesti AM, Spritzer KL: Psychometric properties of the medical outcomes study sleep measure. Sleep medicine. 2005, 6:41–44.
Allen RP, Kosinski M, Hill-Zabala CE, Calloway MO: Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep). Sleep medicine. 2009, 10:531–539.
Stepnowsky C, Johnson S, Dimsdale J, Ancoli-Israel S: Sleep apnea and health-related quality of life in African-American elderly. Annals of behavioral medicine. 2000, 22:116–120.
Kirby KN, Petry NM, Bickel WK: Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental psychology: general. 1999, 128:78.
Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J: Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction. 2016.
Brener ND, Billy JO, Grady WR: Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: Evidence from the scientific literature. Journal of adolescent health. 2003, 33:436–457.
Wagenknecht LE, Cutter GR, Haley NJ, et al.: Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. American journal of public health. 1990, 80:1053–1056.
Muthén LK, Muthén BO: Mplus user’s guide: Statistical analysis with latent variables: User’s guide: Muthén & Muthén, 2010.
Fritz MS, MacKinnon DP: Required sample size to detect the mediated effect. Psychological science. 2007, 18:233–239.
Tofighi D, MacKinnon DP: RMediation: An R package for mediation analysis confidence intervals. Behavior research methods. 2011, 43:692–700.
Hu Lt, Bentler PM: Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal. 1999, 6:1–55.
Sacks V, Murphey D, Moore K: Adverse childhood experiences: National and state-level prevalence. 2014.
Pechtel P, Pizzagalli DA: Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011, 214:55–70.
Ford ES, Anda RF, Edwards VJ, et al.: Adverse childhood experiences and smoking status in five states. Preventive Medicine. 2011, 53:188–193.
Williams DR, Mohammed SA, Leavell J, Collins C: Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010, 1186:69–101.
Chapman DP, Liu Y, Presley-Cantrell LR, et al.: Adverse childhood experiences and frequent insufficient sleep in 5 US States, 2009: A retrospective cohort study. BMC public health. 2013, 13:3.
Wong MM, Brower KJ, Zucker RA: Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep medicine. 2009, 10:787–796.
Chee MW, Chuah LY: Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Current opinion in neurology. 2008, 21:417–423.
Gujar N, Yoo S-S, Hu P, Walker MP: Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. The Journal of Neuroscience. 2011, 31:4466–4474.
Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM: The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep medicine reviews. 2010, 14:179–189.
De Wit H: Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction biology. 2009, 14:22–31.
Kerkhof G, Van Dongen H: Effects of sleep deprivation on cognition. Human Sleep and Cognition: Basic Research. 2010, 185:105.
Musiek ES, Holtzman DM: Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016, 354:1004–1008.
Odum AL, Baumann AA: Delay discounting: State and trait variable. 2010.
Koffel E, Watson D: The two-factor structure of sleep complaints and its relation to depression and anxiety. Journal of abnormal psychology. 2009, 118:183.
Edwards VJ, Anda RF, Gu D, Dube SR, Felitti VJ: Adverse childhood experiences and smoking persistence in adults with smoking-related symptoms and illness. The Permanente Journal. 2007, 11:5–13.
Colrain IM, Trinder J, Swan GE: The impact of smoking cessation on objective and subjective markers of sleep: Review, synthesis, and recommendations. Nicotine & tobacco research. 2004, 6:913–925.
MacKillop J, Amlung MT, Few LR, et al.: Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology. 2011, 216:305–321.
Acknowledgements
This research was supported by grants R01 DA029488 and P30 DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. Dr. MacKillop’s contributions were partially supported by the Peter Boris Chair in Addictions Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards: Authors Oshri, Kogan, Liu, Sweet, and MacKillop declare that they have no conflicts of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
About this article
Cite this article
Oshri, A., Kogan, S., Liu, S. et al. Pathways Linking Adverse Childhood Experiences to Cigarette Smoking Among Young Black Men: a Prospective Analysis of the Role of Sleep Problems and Delayed Reward Discounting. ann. behav. med. 51, 890–898 (2017). https://doi.org/10.1007/s12160-017-9914-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-017-9914-0