Abstract
Background
Sexual minorities have documented elevated risk factors that can lead to inflammation and poor immune functioning.
Purpose
This study aims to investigate disparities in C-reactive protein (CRP) and Epstein–Barr virus (EBV) by gender and sexual orientation.
Methods
We used the National Longitudinal Study of Adolescent Health to examine disparities in CRP (N = 11,462) and EBV (N = 11,812).
Results
Among heterosexuals, women had higher levels of CRP and EBV than men. However, sexual minority men had higher levels of CRP and EBV than heterosexual men and sexual minority women. Lesbians had lower levels of CRP than heterosexual women.
Conclusions
Gender differences in CRP and EBV found between men and women who identify as 100 % heterosexual were reversed among sexual minorities and not explained by known risk factors (e.g., victimization, alcohol and tobacco use, and body mass index). More nuanced approaches to addressing gender differences in sexual orientation health disparities that include measures of gender nonconformity and minority stress are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
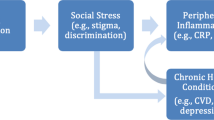
Numerous studies indicate that lesbians, gays, bisexuals, and individuals who identify as “mostly heterosexual” (sexual minorities) experience increased exposure to victimization and discrimination, both interpersonally and institutionally [1–5]. Indeed, the minority stress paradigm posits that the elevated levels of mental health disorders and risk behaviors observed among the sexual minority population are a direct result of the chronic exposure to stigma and increased likelihood of experiencing discrimination and victimization [6, 7]. As a result of increased stress and subsequent engagement in risk behaviors, research has documented that sexual minority populations are more likely to report poorer general health [8] and chronic morbidities [9, 10], as well as increased cardiovascular disease risk [11, 12]. Previous work has established a relationship between stress exposure and poor immune functioning and cell-mediated immunity in the general population [13, 14], but the implications for sexual minority health remains uncertain. Understanding the underlying biological mechanisms that link sexual orientation to poor physical health is an important and understudied field of inquiry.
Existing research on gender differences in inflammation and immune functioning has demonstrated that, compared with men, women have higher levels of inflammation and poorer immune functioning [15–19]. Given the growing body of work that suggests higher rates of gender nonconforming expression among the sexual minority population [20–22], it is unclear whether these disparities generalize to sexual minority men and women. To be sure, while among both heterosexual and sexual minority populations, a wide range of gender presentations, attitudes, and behaviors exist, at the population level research suggests that sexual minority men and women are more likely to exhibit gendered behaviors, attitudes, or personality characteristics nonconforming to those found among heterosexual peers of the same sex. It is unknown if the gender nonconformity observed in attitudes and behaviors extends to biological markers of inflammation and cell-mediated immunity.
Thus, this paper investigates two competing hypotheses. First, that under minority stress theory all sexual minorities will have higher levels of inflammation and poorer immune functioning than do heterosexuals. By comparison, if gender nonconformity extends to physiological indicators, we would observe elevated levels of inflammation and poorer immune functioning among sexual minority men compared with heterosexual men but the reverse among women: sexual minority women would have lower levels of inflammation and better immune functioning than heterosexual women.
Stress and Inflammation and Immune Functioning
Both C-reactive protein (CRP) and Epstein–Barr virus (EBV) are sensitive, at least in the short term, to the effects of psychosocial stressors. As such, they have been posited to be one pathway through which stressors get “under the skin” to influence physical health [13, 23]. When individuals experience psychosocial stressors, a series of biological responses are triggered that include activation of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis. Stress response system activation can have downstream influences on the immune system, such as triggering increases in markers of systemic inflammation, such as CRP [14, 24–26]. CRP has important implications for overall health, in particular cardiovascular disease (CVD) [27–29] and was recently recommended by the American Heart Association to be monitored as a risk factor for CVD [30].
Stress exposure also influences immune functioning through indirect pathways involving health behaviors [31]. Indeed, coping strategies such as disordered eating and binge drinking are both related to elevations in CRP [32–34]. Smoking has been shown to suppress immune functioning [25, 35], which has important long-term implications for infection and illness. Obesity has also been linked to low-level inflammation via the relationship between adipose tissue and increased production of cytokines and hormones, such as cytokine interleukin 6 [36] and leptin [37].
EBV links stress exposure and cell-mediated immunity [23, 38–40]. Roughly 80–90 % of the US population is EBV seropositive [41]. Once acquired, the virus remains in the body for life, but usually in a latent or inactive state. During periods of stress, immune dysregulation can lead to reactivation of the virus as evidenced by higher levels of EBV titers. The higher levels of EBV antibodies reflect a failure of the immune system to regulate the virus. EBV as an indicator of cell-mediated immunity has been linked to a variety of negative health outcomes including cancer [42], wound healing [43], and multiple sclerosis [44, 45].
Under the minority stress framework, increased exposure to victimization and discrimination experienced by sexual minorities might trigger elevated levels of both CRP and EBV among sexual minority men and women relative to heterosexuals. Previous research has shown that exposure to stress, such as victimization and discrimination, as well as its potential outcomes (e.g., depression) are linked to CRP and EBV [38, 46–48]. Moreover, several studies have demonstrated that sexual minorities are more likely to report a variety of risk indicators related to exposure to minority stress and compromised immune functioning, including tobacco and alcohol misuse [49–51], as well as risky dieting behaviors and obesity [52–54]. The direct effect of stress exposure on inflammation and cell-mediated immunity, coupled with behavioral risk factors, may translate to disparities by sexual orientation in inflammation and cell-mediated immunity. Moreover, observed disparities would be expected to be partially, if not fully, mediated by known risk factors associated with stress.
Gender Differences in Inflammation and Immune Functioning
Extensive research has documented gender differences in inflammation and immune functioning related to biological differences between men and women [18, 55–59]. Women exhibit greater changes in immune functioning in response to psychosocial stressors than men [15, 16, 18, 19]. This difference in immune responses is advantageous to women’s health for combating infection but could be detrimental in the case of chronic inflammation. As a result, several studies have demonstrated that women have higher levels of CRP compared with men [55–57, 60], suggesting elevated systemic inflammation. Few studies have examined gender differences in cell-mediated immune processes, such as titers to latent infections like EBV. The existing research suggests that there may be gender differences in EBV levels as a response to stressors [61] and that there are gender differences in other markers of cell-mediated immunity including cytomegalovirus and herpes simplex virus-1 [16, 62, 63]. The differences in immune functioning between men and women have been attributed to a variety of factors. Some research has suggested that differences in inflammation and immune functioning may be related to sex hormones, in particular, estrogen [64, 65]. Differences in adiposity have also been suggested as a reason for women’s higher levels of CRP. Obesity has been linked to higher levels of inflammation [66] and the relationship between fat, in both quantity and distribution, has a stronger effect on women’s CRP than men’s.
In addition to biological factors, differences in immune functioning have also been linked to gender differences in both the perception of and reactions to stress. Compared with men, women are more likely to report perceiving life events as stressful [67–71] and are less likely to engage in active problem solving strategies [72, 73]. Instead, compared with men, women are more likely to internalize stress and engage in rumination, or the tendency to focus on a specific problem or source of stress in the absence of active problem solving techniques [74, 75]. Engagement in rumination has repeatedly been linked to anxiety and depression and is key mediator of gender disparities in depressive symptoms [73–75]. These differences in coping mechanisms may have important implications for gender differences in inflammation and immune functioning through their influence on mental health outcomes, like anxiety and depression, which have been linked to inflammation and immune functioning [76–78].
Most of the studies on gender differences in stress and stress responses, both behavioral and physiological, have been conducted with heterosexual samples. However, the degree to which observed patterns generalize to sexual minorities remains to be determined. Research shows that gender nonconformity is prevalent among sexual minorities, both in retrospective and prospective studies [20, 21]. For example, sexual minority men are more likely to engage in feminine-type play and dress in childhood [79, 80]. The reverse is true for sexual minority women; lesbians are more likely to recall being called tomboys and engaging in more masculine-type play and dress [81, 82]. Gender nonconformity among sexual minorities has been found not only in gender presentation during both childhood and adulthood [20, 21, 79, 80] but also in a variety of other situations including occupational and activity interests [22, 81] and health care seeking behaviors [82]. Moreover, a recent meta-analysis showed that sexual minority men and women were also gender nonconforming in several personality characteristics: gay women scored lower on neuroticism and higher on instrumentality than heterosexual women, whereas gay men scored higher on agreeableness, expressiveness, and neuroticism than heterosexual men [22], which have implications for coping behaviors [83].
Other work has shown that there are gender differences in health behaviors related to inflammation by sexual orientation. In particular, studies have shown that sexual minority women are more likely to engage in smoking and hazardous drinking compared with heterosexual women [50, 84, 85], but these differences are smaller among men [84, 85]. Research has also consistently shown that sexual minority women are more likely to be obese compared with heterosexual women [53, 54], while gay men have lower body mass indexes (BMIs) than heterosexual men [86].
Two recent studies provide conflicting results for the relationship between sexual minority status and inflammation. While one study suggested that sexual minority men had elevated levels of CRP compared with heterosexuals [12], the other study found lower levels of inflammation, measured as part of a composite cardiovascular health scale called allostatic load, among gay and bisexual men [87]. If gender nonconformity holds across biological indicators, we would expect to see that sexual minority women have lower levels of inflammation and better immune functioning than heterosexual women, whereas sexual minority men have higher levels of inflammation and poorer immune functioning than heterosexual men. An extension of this hypothesis would posit that sexual minority women are more similar in inflammation and immune functioning to heterosexual men, and sexual minority men are more similar on these physical health markers to heterosexual women.
Present Study
Studies consistently show gender differences in inflammation and immune functioning among heterosexual populations. These differences exist for a variety of reasons, both biological and behavioral. Although prior research has examined sexual orientation disparities in risk factors for inflammation, to date, few studies have examined disparities in CRP and EBV by sexual orientation and gender. Thus, this study examines three primary research questions. First, are there differences in CRP and EBV risk factors associated with minority stress by sexual orientation and gender? Second, do disparities exist in CRP and EBV by sexual orientation and gender; and if so, are these disparities explained by known risk factors associated with minority stress? Third, does gender nonconformity among sexual minorities extend to physiological measures? Existing research shows that heterosexual women have higher levels of inflammation and immune dysfunction than men. The gender nonconforming hypothesis would suggest a reversed gendered pattern among sexual minority men and women: sexual minority men would have higher levels of CRP and EBV than heterosexual men and sexual minority women would have lower levels than heterosexual women.
Methods
This study uses data from the National Longitudinal Study of Adolescent Health (Add Health), a nationally representative longitudinal study of US men and women. The initial Add Health sample was drawn from 80 high and 52 middle schools throughout the USA, with unequal probabilities of selection [88]. The first wave of the Add Health study (1994/1995) surveyed 90,118 adolescents who filled out a brief in-school survey. A subsample of students (n = 20,747) was asked to fill out an additional in-depth home interview survey. This study used Wave IV of the Add Health survey, between 2007 and 2008, which included 80.3 % of the eligible Wave I in-home sample respondents. At the time of the Wave IV interview, respondents completed an in-depth survey and provided numerous biological samples, including blood spot data that was later assayed for CRP and EBV. Our sample is therefore restricted to respondents surveyed in Wave IV who participated in bio-specimen data collection (N = 14,049). Respondents with CRP and/or EBV measures outside of expected high ranges or large differences in duplicate specimens were flagged and coded as missing. CRP is a relatively stable measure of inflammation [89]; however, it is responsive to environmental stressors [24, 90]. CRP’s responsiveness to social and environmental stressors is in line with our goal of identifying risk factors associated with inflammation. In line with clinical recommendations, we restrict our analysis to persons with CRP levels below 10 mg/l whose elevated levels of CRP would be indicative of infection [30]. For the analysis of EBV, we exclude individuals with scores below the 10th percentile to reflect previously established trends in EBV seronegativity [91]. Sensitivity analyses conducted using a 15th percentile cutoff yielded similar results. For the sexual orientation item, we excluded respondents who answered “don’t know,” that they were “not sexually attracted to either males or females,” or refused to answer the sexual orientation identity survey item. We also excluded individuals who reported having tested positive for HIV (n = 18). Our final sample size was 11,462 for the analysis of CRP, of which 46.9 % were female and the mean age was 28.8 years (SD = 0.12). For the analysis of EBV, the final sample size was 11,812, of which 51.3 % were female and the mean age was 28.8 years (SD = 0.12).
To be sure we were not introducing bias to our results we also performed supplementary analyses that restricted the sample to respondents who were included in both the CRP and EBV samples. The results revealed that the overall trends were the same; however, statistical significance was affected because of the large reduction in sample size, particularly for the already small samples of some sexual minority groups. We also investigated whether there may be bias in the CRP and EBV samples by sexual orientation or gender, our main predictor variables. We found that there were no significant differences in the distribution of sexual orientation across both samples, nor were there biases in the gender distribution.
Measures
Biomarkers
In Wave IV at the time of interview, blood spot samples were obtained using a finger prick and collected on standardized filter paper using a sterile lancet. Blood spots were dried overnight and then frozen prior to laboratory analysis. High sensitivity CRP (milligrams per liter) was assayed from blood spots using a standardized enzyme immunoassay protocol. EBV (arbitrary units per milliliter) was assayed using an adaptation of a previously validated protocol [62]. Previous validation studies indicate a high correlation between values from blood serum and blood spot samples for both CRP and EBV [61, 92]. To approximate normal distributions, both CRP and EBV were log transformed. Logged CRP had a mean of 0.79 (SD = 0.02) and ranged from −6.21 to 2.30, and logged EBV had a mean of 4.94 (SD = 0.01) and ranged from 3.87 to 7.18.
Predictors
Gender was measured using a single item from Wave IV that asked respondents to identify as male (referent) or female. Sexual orientation was measured using an item from Wave IV that asked respondents whether they identify as “100 % heterosexual; mostly heterosexual; bisexual; mostly gay; or 100 % gay.” From this item, a series of four dummy variables were created to measure sexual orientation: 100 % heterosexual (referent), mostly heterosexual, bisexual, and mostly gay/100 % gay. Preliminary results showed that mostly gay and 100 % gay respondents did not differ for either measure and thus they were combined.
Covariates
Psychosocial Stressors
This study included several measures of stress and stress responses that are elevated among the sexual minority population and linked to compromised immune functioning including victimization and depression [6, 7, 93].
Childhood physical abuse was derived from an item that asked respondents, “By the time you started 6th grade, how often had your parents or other adult caregivers slapped, hit or kicked you?” Respondents who reported five or more incidents were coded as yes, those who reported no incidences or less than five were coded as no (referent), and respondents who refused or skipped the question were coded as missing.
Forced sex was measured in Wave IV using two survey items that asked respondents if they have “ever been forced, in a nonphysical way, to have any type of sexual activity against your will? For example, through verbal pressure, threats of harm or by being given alcohol or drugs” and “Have you ever been physically forced to have any type of sexual activity against your will?” These two questions specifically exclude experiences with a parent or adult caregiver. Respondents who reported either nonphysical or physical sexual coercion were coded as yes [1] and those who did not were coded as no (0, referent).
Physical victimization in the previous 12 months was coded as a binary variable based on a Wave IV item that asked “which of the following things happened in the last month: someone pulled a knife or gun on you; someone shot or stabbed you; someone slapped, hit, choked, or kicked you; you were beaten up?” Respondents who reported at least one of these incidents were coded as having been victimized.
Discrimination was measured in Wave IV with the question “In your day-to-day life, how often do you feel you are treated with less respect or courtesy than other people? … Do you think the main reason for these experiences was your sexual orientation?” We created a dichotomous variable that captures whether respondents report being treated with less respect due to their sexual orientation never or rarely (referent) versus sometimes or often.
Perceived stress in Wave IV was measured using the Cohen Perceived Stress scale [94]. The total scale ranges from 0 to 16 and had an alpha of 0.72 in the analytic sample. Stress response in Wave IV is measured using the depressive symptoms scale of the abbreviated Center for Epidemiologic Studies Depression Scale (CES-D). The CED-D scale ranges from 0 to 20 [95], and had a Chronbach’s α of 0.79 in our analytic sample.
Risk Indicators
Several measures of risk indicators were included that are elevated among sexual minority population and linked to inflammation and immune functioning including tobacco and alcohol use [49–51], as well as obesity [52–54].
The tobacco use at Wave IV measured whether respondents were current regular smokers, operationalized as at least one cigarette a day for 30 days, former regular smokers, or never regular smokers (referent). Binge drinking was derived from a survey item that asked respondents, “During the past 12 months, on how many days did you drink 5 or more (if male) or 4 or more (if female) drinks in a row?” This measure was coded as a continuous measure that ranges from zero to six, where zero is equal to no episodes of binge drinking and six is equal to every day or almost every day.
Anthropometric measures of height and weight were taken at the time of interview in Wave IV and used to calculate BMI kg/m2 for respondents. We used the World Health Organization (WHO) obesity classifications [96] to measure whether respondents were underweight (BMI < 18), healthy weight (BMI ≥ 18 and <25), overweight (BMI ≥ 25 and ≤30), obese class I (BMI > 30 and ≤35), or obese class II/III (BMI >35) (referent).
Physical activity was derived from a series of questions that asked respondents how many times in the past week they engaged in a variety of physical activities such as bicycling, skateboarding, hiking, roller blading, team sports, aerobics, individual sports, weight training, or walking for exercise. The measure is coded as a scale that ranges from zero bouts of activity in the last 7 days to 20 or more.
Statistical Controls
All analyses controlled for race/ethnicity, age, and education. Race/ethnicity was categorized as non-Hispanic white (referent); non-Hispanic black; Hispanic; non-Hispanic Asian; or other. Age was coded as a continuous variable ranging from 24 to 34 years. Education was measured as a series of dummy variables that identified whether respondents had less than a high school education, graduated from high school, had attended some college, or graduated from college or received post-graduate education (referent). We also included a control for prescription hormone use, such as contraception, derived from prescription drug rosters filled out by respondents.
Statistical Analysis
This study used ordinary least squares (OLS) multivariate regression analyses to examine disparities in CRP and EBV by sexual orientation. First, we present descriptive statistics for all variables included in the analysis for the total population and stratified by sexual orientation and gender. We conducted F-tests using the “test” command in Stata 12.0 to test for statistical differences in means for each sexual minority identity compared with 100 % heterosexual respondents, and for differences between men and women. Next, we present the results from our multivariate regressions. For both CRP and EBV, we ran three models. The first examined sexual orientation and gender disparities controlling for basic sociodemographic characteristics of ethnicity/race, age, and educational attainment. The second model added controls for all other potential explanatory factors, and the third model examined the interaction of sexual orientation by gender to test if disparities varied by gender across sexual orientation groups. Finally, we present the results from multivariate models for sexual orientation disparities in CRP and EBV stratified by gender. All analyses used population weights to reflect the US population and the SVY commands in Stata version 12.1 to account for the complex design by adjusting variance estimates.
Results
Differences in CRP and EBV Risk Factors by Sexual Orientation and Gender
There were several differences in inflammation and immune functioning risk factors by sexual orientation shown in Table 1. All mostly heterosexual, bisexual, and gay respondents reported significantly higher levels of stress exposure and depressive symptoms compared with heterosexual respondents. Additionally, compared with heterosexual-identified respondents, bisexual and mostly heterosexual respondents were more likely to be current smokers, and gay respondents reported higher levels of binge drinking and being a victim of forced sex. Gay respondents were also more likely to report discrimination based upon their sexual orientation compared with heterosexual respondents (1.91 vs. 0.01, p < .001).
Significant gender differences in stress-related and risk factors were also observed. One in four women reported being a victim of forced sex in their lifetime, compared with 4 % of men, but men were more likely to report being physically victimized in the previous 12 months compared with women (24.1 % vs. 18.6 %, p < .05). In line with other research, women also reported higher levels of perceived stress and more depressive symptoms. There were also significant gender differences in other risk factors: men were more likely to report being a current smoker and drinking on a more regular basis than women. A slightly higher percentage of women were in the healthy BMI range of 18 to 25 kg/m2 compared with men, but they were less likely to report high levels of physical activity.
Sexual Orientation and Gender Disparities in CRP and EBV
The results show that among the total population, there were no differences in CRP and EBV by sexual orientation. Table 2 presents the results from multivariate OLS regression models examining the relationships among sexual orientation, gender, and both CRP and EBV. Panel A presents results for CRP and Panel B presents for EBV. The results for Model 1 showed no disparities at baseline, and the inclusion of inflammation risk factors in Model 2 had little to no impact on the estimates for sexual orientation. However, women had higher levels of CRP in both models compared with men, as expected. A similar, but weaker relationship emerged for EBV. There were no differences in EBV by sexual orientation, but women had higher levels of EBV compared with men.
Gender Nonconformity in CRP and EBV Risk Factors Among Sexual Minorities
The results show support for gender nonconformity in CRP and EBV among sexual minorities. This result is particularly strong for the CRP results. This finding is illustrated by both the significant interaction between gender and sexual orientation for CRP, suggesting that gay and bisexual men have higher levels of CRP than heterosexual men, and lesbian and bisexual women have lower levels than heterosexual women. The interactions remain significant even after controlling for several known risk factors, including victimization, stress and depression, alcohol and tobacco use, and BMI. Interestingly, the results also show that within the bisexual and gay respondents, men have higher levels of CRP than women. This is the opposite of the finding among heterosexual respondents. The interaction results are displayed in Table 3.
Similar to CRP, the interactions in Table 2, Model 3 shows changes in gender differences in EBV by sexual orientation. Among heterosexual respondents, women had higher levels of EBV than men, but among gay and lesbian respondents there were no significant differences in EBV.
Follow-up analysis to the interactions is displayed in Table 4, which presents the results from multivariate regressions stratified by gender to further investigate sexual orientation disparities in CRP and EBV. Among women, lesbians had lower levels of CRP compared with heterosexuals (β = −0.33, p < .05), and mostly heterosexual women had lower levels of EBV (β = −0.04, p < .10). Among men, bisexual men had higher levels of CRP (β = 0.64, p < .001) and gay men had higher levels of EBV compared with heterosexuals (β = 0.17, p < .05). Thus, we see opposite patterns of EBV and CRP disparities in sexual orientation by gender. Among women, the results suggest lower levels of inflammation for sexual minorities, whereas among men, we see higher levels of inflammation among sexual minorities.
Additional analysis that also included controls for prescription hormone use did not change the results. Given that BMI is highly related to inflammation, we also conducted additional analysis stratified by weight status. These additional analyses revealed that the same gender and sexual orientation patterns observed in the full cohort were seen among the healthy BMI range of 18 to 25 kg/m2 and among respondents who were overweight or obese (not in tables).
Discussion
This study examines both sexual orientation and gender disparities using biomarkers of inflammation and immune functioning, CRP and EBV. The results provide new insights into the relationship between sexual orientation and important biological processes by demonstrating that the well-documented finding from the prior literature that women have higher levels of inflammation and poorer immune functioning than men does not hold across all sexual orientation groups. Rather, among gay and bisexual respondents, the results show that men have higher levels of CRP and similar levels of EBV to women of comparable sexual orientation. Gay and bisexual men had higher levels of CRP and EBV compared with heterosexual men, whereas sexual minority women in general and lesbian women in particular had lower levels of CRP than heterosexual women and similar levels of CRP compared with heterosexual men. These findings were particularly strong for CRP and were unexplained by many of the prevalent causal mechanisms in the literature related to stressors and stress-related outcomes (e.g., depressive symptoms).
The results presented here suggest that understanding inflammation and immune functioning across sexual orientation groups requires an expanded framework for minority stress that incorporates sex/gender differences in a more nuanced way. Under minority stress theory, we would expect to find that both sexual minority men and women have higher levels of CRP and EBV compared with same-gender heterosexuals as a consequence of elevated levels of exposure to gay-related victimization. Moreover, we would expect that a large portion of this excess risk would be attributable to exposure to stressors and risk-related coping behaviors. Several studies have linked stress to inflammation and immune functioning [13, 14, 23] and documented that sexual minorities are more likely to report a variety of inflammation risk factors [49–53]. Theoretically, one would expect for this to translate into elevated risk of inflammation for both sexual minority men and women. Our results, however, showed that gay men had higher levels of CRP and EBV compared with heterosexual men, whereas lesbians had lower levels of CRP and similar levels of EBV as heterosexual women. This finding supports new research that showed that sexual minority men have higher levels of CRP compared with heterosexual men [12]. In addition, bisexual men had higher levels of CRP than heterosexual women and gay men and heterosexual women had similar levels of EBV. Lesbians, in turn, had levels of CRP and EBV similar to those of heterosexual men. Furthermore, controlling for victimization and other correlates of minority stress had no effect on the relationships between sexual orientation and both CRP and EBV. Rather the sexual orientation disparities in CRP and EBV were moderated by gender. Thus, minority stress theory is not sufficient on its own to explain the observed disparities.
The results revealed that the repeated finding that women have higher levels of CRP than men was reversed among sexual minority men and women, providing support for the gender nonconformity hypothesis. The gender differences observed might be due to a variety of factors. First, studies have shown that sexual minority men are more likely to report experiencing violence and property crimes than sexual minority women [2], and gender nonconforming boys are more likely to face peer rejection than gender nonconforming girls [97]. Moreover, at least one study has shown that gender nonconformity is related to poorer well-being among men, but not among women [98]. Other work, however, has shown that both gender nonconforming girls and boys are both exposed to high levels of victimization [99–101]. However, if sexual minority men did experience higher rates of victimization than sexual minority women, this would not explain why sexual minority women have lower levels of inflammation than heterosexual women.
Thus, in addition to or despite potential differences in stress exposure, it may be that gender nonconforming responses to stress are underlying mechanisms that help to explain our findings. Several studies have shown that there are gender differences in perceived stress and its psychological sequalae [71, 73, 74]. These differences have been shown to play an important role in gender differences in depression and anxiety, both linked to inflammation functioning [76–78]. In a review of sexual orientation and personality characteristics, it was found that gay men and women were more likely to report gender nonconforming personality traits related to coping behaviors [22]. These differences in personality characteristics may in part explain the observed reversal in the gender disparity in inflammation. Indeed, sexual minority women who are gender nonconforming in personality characteristics (e.g., less neuroticism and openness, but more instrumentality) may take more active problem-solving approaches to stressors [83], resulting in better immune functioning. Other research has also suggested that gender nonconformity may benefit women for a variety of outcomes including increased self-efficacy and self-esteem [102–104]. Similar findings have not been found for men. Thus, sexual minority men may have more gender nonconforming responses to stressors that increase inflammation, such as increased rumination, whereas sexual minority women may benefit from gender nonconforming responses to stressors. Given that both sexual minority men and women have elevated levels of exposure to victimization and other stressors, more work is needed to identify gender differences in coping behaviors across sexual orientation groups.
This study has several limitations. First, we are unable to assess gender expression and do not have data on several gendered personality characteristics. At the population level, sexual minorities are more likely to be gender nonconforming in their presentation [20–22], which may in part explain the observed disparities. To further test the gender nonconforming hypothesis, future research should collect data not only on gender presentation, but also consider inquiring about gendered personality characteristics and coping strategies. These factors may reveal that gender nonconforming personality characteristics and coping styles contribute to the patterns found in this study. Still another limitation is that we are not able to rule out biological factors that may influence both sexual orientation and inflammation and immune functioning, although we did control for a number of potential biological risk factors (e.g., BMI and tobacco use). Differences in sex hormones (both pre- and postnatal) may influence sexual orientation and gender expression [105], and consequently inflammation and immune functioning. More research, however, is needed to understand the links between sexual orientation, gender, and sex hormones (testosterone, estrogen, estradiol) in adulthood. There exists some evidence that among women, lesbians who identify with more masculine gender identities have higher levels of testosterone compared with lesbians who identify with more feminine gender identities [106], suggestive of a physiological pathway between gender expression and sex hormones, which may have implications for inflammation and cell-mediated immune functioning. This may also explain why larger gender nonconforming disparities are observed for CRP than EBV, as CRP is more closely linked to sex hormones. More work is needed to understand if and how sex hormones vary across sexual orientations.
The study is also limited by the measures of minority stress. We had one measure of sexual orientation specific discrimination that was not significantly related to CRP or EBV. This is most likely due to the fact that respondents were asked to choose one reason for why they thought they experienced poorer treatment: race/ethnicity, gender, age, weight, sexual orientation, or socioeconomic status. Many sexual minority respondents with multiple stigmatized identities may have chosen other reasons, rather than their sexual orientation, as the main reason for why they experienced discrimination.
This limits our ability to formally test the effects of minority stress on CRP and EBV. More research is needed to directly address the effects of minority stress on markers of inflammation and cell-mediated immunity. Moreover, future research may benefit from exploring how multiple minority statuses influence inflammation and immune functioning, in particular those related to race/ethnicity, for which there are documented disparities in CRP and EBV [107–109]. Finally, given the small sample size, the results for bisexual and mostly heterosexual men should be interpreted cautiously.
Despite the limitations, our results provide new insights into the relationship between sexual orientation and inflammation and immune functioning by revealing that the repeated finding that women have higher levels of inflammation compared with men does not hold across all sexual orientation groups. Rather, the reverse is true among bisexual and gay/lesbian-identified persons for CRP and no differences were detected in EBV between sexual minority gay/lesbian respondents. These trends were unexplained by many of the existing stress-related causal mechanisms in the literature. This result highlights the need for continued investigation of the relationship among sex/gender, sexual orientation, and inflammation and immune functioning. This report finds that minority stress theory is an insufficient explanation for the disparities observed in this study. Rather, we find support for the influence of gender nonconformity in inflammation and immune functioning among sexual minorities. Future research should take a more nuanced approach to addressing gender differences in sexual orientation health disparities.
References
Kosciw JG, Greytak EA, Bartkiewicz MJ, Boesen MJ, Palmer NA. The 2011 National School Climate Survey: The experiences of lesbian, gay, bisexual and transgender Youth in Our Nation’s Schools. 2012. Available at http://www.eric.ed.gov/ERICWebPortal/recordDetail?accno=ED535177. Accessed April 10, 2013.
Herek GM. Hate crimes and stigma-related experiences among sexual minority adults in the United States. J Interpers Violence. 2009; 24: 54-74.
Huebner DM, Rebchook GM, Kegeles SM. Experiences of harassment, discrimination, and physical violence among young gay and bisexual men. Am J Public Health. 2004; 94: 1200-1203.
Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011; 127: 896-903.
Hatzenbuehler ML, McLaughlin KA, Keyes KM, Hasin DS. The impact of institutional discrimination on psychiatric disorders in lesbian, gay, and bisexual populations: A prospective study. Am J Public Health. 2010; 100: 452-459.
Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychol Bull. 2003; 129: 674-697.
Meyer IH. Minority stress and mental health in gay men. J Health Soc Behav. 1995; 36: 38-56.
Fredriksen-Goldsen KI, Kim H-J, Barkan SE, Muraco A, Hoy-Ellis CP. Health disparities among lesbian, gay, and bisexual older adults: Results from a population-based study. Am J Public Health. 2013; 103: 1802-1809.
Conron K, Mimiaga M, Landers S. A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health. 2010; 100: 1953-1960.
Cochran SD, Mays VM. Physical health complaints among lesbians, gay men, and bisexual and homosexually experienced heterosexual individuals: Results from the California Quality of Life Survey. Am J Public Health. 2007; 97: 2048-2055.
Everett B, Mollborn S. Differences in hypertension by sexual orientation among U.S. young adults. J Commun Health. 2013; 38: 588-596.
Hatzenbuehler ML, McLaughlin KA, Slopen N. Sexual orientation disparities in cardiovascular biomarkers among young adults. Am J Prev Med. 2013; 44: 612-621.
Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004; 130: 601-630.
McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Ann NY Acad Sci. 1998; 840: 33-44.
Shames RS. Gender differences in the development and function of the immune system. J Adolescent Health. 2002; 30: 59-70.
McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Ex. 2011; 59: 203-213.
Cartier A, Côté M, Lemieux I, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009; 89: 1307-1314.
Casimir GJA, Duchateau J. Gender differences in inflammatory processes could explain poorer prognosis for males. J Clin Microbiol. 2011; 49(1): 478-479.
Cannon JG, St. Pierre BA. Gender differences in host defense mechanisms. J Psychiat Res. 1997; 31(1): 99-113.
Bailey JM, Zucker KJ. Childhood sex-typed behavior and sexual orientation: A conceptual analysis and quantitative review. Dev Psychol. 1995; 31: 43-55.
Zucker KJ. Reflections on the relation between sex-typed behavior in childhood and sexual orientation in adulthood. J Gay Lesbian Mental Health. 2008; 12(1–2): 29-59.
Lippa RA. Sexual orientation and personality. Ann Rev Sex Res. 2005; 16(1): 119-153.
Glaser R, Pearson GR, Jones JF, et al. Stress-related activation of Epstein–Barr virus. Brain Behav Immun. 1991; 5: 219-232.
Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 17(4): 286–295.
Tracy RP, Psaty BM, Macy E, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscl Throm Vas. 1997; 17: 2167-2176.
Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012; 106: 29-39.
Ridker PM. C-reactive protein, metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14,719 initially healthy American women. Circulation. 2003; 107: 391-397.
Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000; 3: 469-476.
Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010; 375: 132.
Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003; 107: 499-511.
Cohen S, Herbert TB. Health psychology: Psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu Rev Psychol. 1996; 47: 113-142.
Alho H, Sillanaukee P, Kalela A, Jaakkola O, Laine S, Nikkari ST. Alcohol misuse increases serum antibodies to oxidized LDL and C-reactive protein. Alcohol Alcoholism. 2004; 39: 312-315.
Bertran N, Camps J, Fernandez-Ballart J, et al. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J Lab Clin Med. 2005; 145: 41-46.
Gentile M, Panico S, Rubba F, et al. Obesity, overweight, and weight gain over adult life are main determinants of elevated HS-CRP in a cohort of Mediterranean women. Eur J Clin Nutr. 2010; 64: 873-878.
Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009; 9: 377-384.
Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999; 106: 506-512.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006; 83: 461S-465S.
Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrino. 2013; 38: 188-200.
Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein–Barr virus in response to examination stress. Psychoneuroendocrino. 1994; 19: 765-772.
Glaser R, Padgett DA, Litsky ML, et al. Stress-associated changes in the steady-state expression of latent Epstein–Barr virus: Implications for chronic fatigue syndrome and cancer. Brain Behav Immun. 2005; 19: 91-103.
Jones JF, Straus SE. Chronic Epstein–Barr virus infection. Annu Rev Med. 1987; 38: 195-209.
Thompson MP, Kurzrock R. Epstein–Barr virus and cancer. Clin Cancer Res. 2004; 10: 803-821.
Godbout JP, Glaser R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. J Neuroimmune Pharm. 2006; 1: 421-427.
Ascherio A, Munch M. Epstein–Barr virus and multiple sclerosis. Epidemiology. 2000; 11: 220-224.
Ascherio A, Munger KL, Lennette ET, et al. Epstein–Barr virus antibodies and risk of multiple sclerosis. JAMA-J Am Med Assoc. 2001; 286: 3083-3088.
Hiles SA, Baker AL, de Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: A meta-analysis. Psychol Med. 2012; 42: 2015-2026.
Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009; 71: 243-250.
Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biolo Psychiat. 2012; 71: 15-21.
Lee JGL, Blosnich JR, Melvin CL. Up in smoke: Vanishing evidence of tobacco disparities in the Institute of Medicine’s report on sexual and gender minority health. Am J Public Health. 2012; 102: 1-3.
Hughes T. Alcohol use and alcohol-related problems among sexual minority women. Alcoholism Treat Quart. 2011; 29: 403-435.
McCabe SE, Hughes TL, Bostwick WB, West BT, Boyd CJ. Sexual orientation, substance use behaviors and substance dependence in the United States. Addiction. 2009; 104: 1333-1345.
French SA, Story M, Remafedi G, Resnick MD, Blum RW. Sexual orientation and prevalence of body dissatisfaction and eating disordered behaviors: A population-based study of adolescents. Int J Eat Disorder. 1996; 19: 119-126.
Austin SB, Nelson LA, Birkett MA, Calzo JP, Everett B. Eating disorder symptoms and obesity at the intersections of gender, ethnicity, and sexual orientation in US high school students. Am J Public Health. 2013; 2: e16-e22.
Boehmer U, Bowen DJ, Bauer GR. Overweight and obesity in sexual-minority women: Evidence from population-based data. Am J Public Health. 2007; 97: 1134-1140.
Lakoski SG, Cushman M, Criqui M, et al. Gender and C-reactive protein: Data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006; 152: 593-598.
Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005; 46: 464-469.
Saltevo J, Vanhala M, Kautiainen H, Kumpusalo E, Laakso M. Gender differences in C-reactive protein, interleukin-1 receptor antagonist and adiponectin levels in the metabolic syndrome: A population-based study. Diabetic Med. 2008; 25: 747-750.
Valentine RJ, McAuley E, Vieira VJ, et al. Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults. Brain Behav Immun. 2009; 23: 643-648.
Wang TN, Lin MC, Wu CC, et al. Role of gender disparity of circulating high-sensitivity C-reactive protein concentrations and obesity on asthma in Taiwan. Clin Exp Allergy. 2011; 41: 72-77.
Ishii S, Karlamangla AS, Bote M, et al. Gender, obesity and repeated elevation of C-reactive protein: Data from the CARDIA cohort. PLoS ONE. 2012; 7(4): e36062.
McDade TW, Stallings JF, Angold A, et al. Epstein–Barr virus antibodies in whole blood spots: A minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000; 62: 560-568.
Villacres MC, Longmate J, Auge C, Diamond DJ. Predominant type 1 CMV-Specific memory T-helper response in humans: Evidence for gender differences in cytokine secretion. Hum Immunol. 2004; 65: 476-485.
Green MS, Cohen D, Slepon R, Robin G, Wiener M. Ethnic and gender differences in the prevalence of anti-cytomegalovirus antibodies among young adults in Israel. Int J Epidemiol. 1993; 22: 720-723.
Skouby SO, Gram J, Andersen LF, Sidelmann J, Petersen KR, Jespersen J. Hormone replacement therapy: Estrogen and progestin effects on plasma C-reactive protein concentrations. Am J Obstet Gynecol. 2002; 186: 969-977.
Cushman M. Effects of hormone replacement therapy and estrogen receptor modulators on markers of inflammation and coagulation. Am J Cardiol. 2002; 90: F7-F10.
Yudkin JS, Stehouwer C, Emeis J, Coppack S. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscl Throm Vas. 1999; 19: 972-978.
Hewitt PL, Flett GL, Mosher SW. The perceived stress scale: Factor structure and relation to depression symptoms in a psychiatric sample. J Psychopath Behav. 1992; 14: 247-257.
Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001; 37: 404-416.
Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008; 115: 291-313.
Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Dev. 1999; 70: 660-677.
Piko B. Gender differences and similarities in adolescents’ ways of coping. Psychol Rec. 2011; 51: 223-235.
Leadbeater BJ, Kuperminc GP, Blatt SJ, Hertzog C. A multivariate model of gender differences in adolescents’ internalizing and externalizing problems. Dev Psychol. 1999; 35: 1268-1282.
Tamres LK, Janicki D, Helgeson VS. Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Pers Soc Psychol Rev. 2002; 6: 2-30.
Nolen-Hoeksema S. Emotion regulation and psychopathology: The role of gender. Ann Rev Clin Psycho. 2012; 8: 161-187.
Nolen-Hoeksema S, Aldao A. Gender and age differences in emotion regulation strategies and their relationship to depressive symptoms. Pers Indiv Differ. 2011; 51: 704-708.
Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003; 65: 347-356.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009; 71: 171-186.
Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004; 164: 1010-1014.
D’augelli AR, Grossman AH, Starks MT. Gender atypicality and sexual orientation development among lesbian, gay, and bisexual youth. J Gay Lesbian Mental Health. 2008; 12: 121-143.
Rieger G, Linsenmeier JAW, Gygax L, Bailey JM. Sexual orientation and childhood sex atypicality: Evidence from home movies. Dev Psychol. 2006; 44: 46-58.
Lippa RA. Sex Differences and sexual orientation differences in personality: Findings from the BBC internet survey. Arch Sex Behav. 2008; 37: 173-187.
Everett BG, Mollborn S. Examining sexual orientation disparities in unmet medical needs among men and women. Popul Res Policy Rev. 2013: e1–25.
Carver CS, Connor-Smith J. Personality and coping. Annu Rev Psychol. 2010; 61: 679-704.
Rosario M, Hunter J, Gwadz M. Exploration of substance use among lesbian, gay, and bisexual youth prevalence and correlates. J Adolescent Res. 1997; 12: 454-476.
Marshal MP, Friedman MS, Stall R, et al. Sexual orientation and adolescent substance use: A meta-analysis and methodological review. Addiction. 2008; 103: 546-556.
Austin SB, Ziyadeh NJ, Corliss HL, et al. Sexual orientation disparities in purging and binge eating from early to late adolescence. J Adolescent Health. 2009; 45: 238-245.
Juster R-P, Smith NG, Ouellet É, Sindi S, Lupien SJ. Sexual orientation and disclosure in relation to psychiatric symptoms, diurnal cortisol, and allostatic load. Psychosom Med. 2013; 75: 103-116.
Bearman PS, Jones J, Udry JR. The National Longitudinal Study of Adolescent Health: Research Design. Available at http://www.cpc.unc.edu/projects/addhealth/design.html. Accessed September 1, 2013.
Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001; 47: 444-450.
Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiat. 2006; 60: 819-824.
Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein–Barr virus infection in US children ages 6–19, 2003–2010. PLoS One. 2013; 8: e64921.
McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004; 50: 652-654.
Katz-Wise SL, Hyde JS. Victimization experiences of lesbian, gay, and bisexual individuals: A meta-analysis. J Sex Res. 2012; 49: 142-167.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983; 24: 385-396.
Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Apl Psych Meas. 1977; 1: 385-401.
World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series no. 894. WHO: Geneva. 2000.
Wallien MSC, Veenstra R, Kreukels BPC, Cohen-Kettenis PT. Peer group status of gender dysphoric children: A sociometric study. Arch Sex Behav. 2010; 39: 553-560.
Skidmore WC, Linsenmeier JA, Bailey JM. Gender nonconformity and psychological distress in lesbians and gay men. Arch Sex Behav. 2006; 35: 685-697.
Roberts AL, Rosario M, Slopen N, Calzo JP, Austin SB. Childhood gender nonconformity, bullying victimization, and depressive symptoms across adolescence and early adulthood: An 11-year longitudinal study. J Am Acad Child Psy. 2013; 52: 143-152.
Roberts AL, Rosario M, Corliss HL, Koenen KC, Austin SB. Elevated risk of posttraumatic stress in sexual minority youths: Mediation by childhood abuse and gender nonconformity. Am J Public Health. 2012; 102: 1587-1593.
Roberts AL, Rosario M, Corliss HL, Koenen KC, Austin SB. Childhood gender nonconformity: A risk indicator for childhood abuse and posttraumatic stress in youth. Pediatrics. 2012; 129: 410-417.
Impett EA, Sorsoli L, Schooler D, Henson JM, Tolman DL. Girls’ relationship authenticity and self-esteem across adolescence. Dev Psychol. 2008; 44: 722-732.
Thornton B, Leo R. Gender typing, importance of multiple roles, and mental health consequences for women. Sex Roles. 1992; 27: 307-317.
Wong PT, Kettlewell G, Sproule CF. On the importance of being masculine: Sex role, attribution, and women’s career achievement. Sex Roles. 1985; 12: 757-769.
Rosario M, Scrimshaw E. Theories and Etiologies of Sexual Orientation. In: APA Handbook of Sexuality and Psychology: Vol 1. Person-based approaches. Washington, DC: American Psychological Association
Pearcey SM, Docherty KJ, Dabbs JM Jr. Testosterone and sex role identification in lesbian couples. Physiol Behav. 1996; 60: 1033-1035.
Cunningham TJ, Seeman TE, Kawachi I, et al. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc Sci Med. 2012; 75: 922-931.
Ford JL, Stowe RP. Racial–ethnic differences in Epstein–Barr virus antibody titers among U.S. children and adolescents. Ann Epidemiol. 2013; 23: 275-280.
Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Ann Epidemiol. 2010; 20: 617-628.
Aknowledgements
This study is supported by NICHD grant R03 HD062597 and by the Office of Research on Women’s Health (ORWH), NICHD grant K12HD055892, and by the University of Colorado Population Center (grant R24 HD066613) through administrative and computing support. SB Austin is supported by NICHD grant R01 HD066963 and Maternal and Child Health Bureau, Health Resources and Services Administration, training grants MC00001 and Leadership Education in Adolescent Health Project 6T71-MC00009. K McLaughlin is supported by NIH grant K01-MH092526. The authors thank the anonymous reviewers for their insightful and helpful comments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the CDC, or any agencies involved in collecting the data.
Conflict of Interest
The authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Everett, B.G., Rosario, M., McLaughlin, K.A. et al. Sexual Orientation and Gender Differences in Markers of Inflammation and Immune Functioning. ann. behav. med. 47, 57–70 (2014). https://doi.org/10.1007/s12160-013-9567-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-013-9567-6