Abstract
Background
Omega-3 fatty acids reduced heart rate (HR) and blood pressure (BP) in some studies, but dose–response studies are rare, and little is known about underlying mechanisms.
Purpose
We examined effects of 0.85 g/day eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) (low dose) and 3.4 g/day EPA + DHA (high dose) on HR and systemic hemodynamics during rest, speech, and foot cold pressor tasks.
Methods
This was a dose–response, placebo-controlled, double-blind, randomized, crossover trial (8-week treatment, 6-week washout) in 26 adults.
Results
Throughout the testing sessions, HR was reduced in a dose-dependent manner. The high dose reduced BP and stroke volume and increased pre-ejection period. Reductions in BP were associated with increases in erythrocyte omega-3 fatty acids.
Conclusions
High-dose long-chain omega-3 fatty acids can reduce BP and HR, at rest and during stress. These findings suggest that at-risk populations may achieve benefits with increased omega-3 intake.
The trial was registered on ClinicalTrials.gov (NCT00504309).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevated blood pressure (BP) and heart rate (HR) are risk factors for heart disease and stroke [1–3]. The omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) appear to reduce risk of cardiovascular death, at least partially via anti-arrhythmic mechanisms [4, 5]. However, their effects on heart rate and blood pressure, particularly under psychological/physiologic stress, are less well understood.
Meta-analyses demonstrate that omega-3 fatty acids reduce resting HR [6] and BP [7–9]. Reductions in BP are more evident in people with hypertension [10–12], and may be dependent on dose [7] and attendant changes in plasma phospholipid omega-3 content [13]. Testing protocols have ranged from one to three resting measures to 24 h ambulatory monitoring with measurements recorded every 30 min [6]. Changes in cardiovascular physiology may be more evident under conditions of stress due to beneficial effects of omega-3 fatty acids on autonomic tone, as evidenced by reductions in plasma norepinephrine during stress [14–17] and improved HR variability [18, 19]. In a recent study of 34 healthy, young participants randomly assigned to a high EPA fish oil supplement (1 g/day EPA + 0.4 g/day DHA) or corn oil placebo for 21 days, blood pressure responses to mental arithmetic were significantly attenuated without effects on resting parameters [20]. Furthermore, we have shown that plant-derived omega-3 fatty acids significantly reduce blood pressure during stress [21]. However, controls in prior studies have been inadequate, limiting interpretation of their findings.
Recommendations for omega-3 intake for CVD prevention typically do not exceed 1 g/day EPA + DHA [22]; yet HR and BP reductions have been reported more consistently at higher (pharmacologic) doses, i.e., 3.4 g/day EPA + DHA [6, 9] which is the approved dose for the treatment of severe hypertriglyceridemia (fasting triglycerides ≥500 mg/dl). Owing to the absence of relevant dose–response studies, we compared the effects of 0.85 g/day EPA + DHA (low dose) and 3.4 g/day EPA + DHA (high dose) on hemodynamic measures during a repeated measures design that included rest, standardized mental stress (speech task), and physical challenge (foot cold pressor).
We hypothesized that the high but not low dose would reduce BP and HR values during testing sessions. Our secondary aim evaluated whether changes in BP and HR were related to changes in erythrocyte membrane omega-3 fatty acid content. Analyses were conducted to characterize other effects of omega-3 fatty acids on systemic hemodynamics (total peripheral resistance, stroke volume, and contractility indexes). Previously, we reported the effects of these treatments on lipids, glycemic markers, endothelial function, and inflammatory markers [23].
Methods
Participants
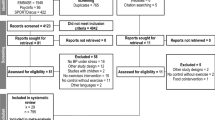
Healthy people (23 men and three women) with moderate hypertriglyceridemia (fasting triglycerides 150–500 mg/dL) were recruited for this study [23]. All women were post-menopausal (no menses >12 months). Other inclusion criteria were: age 21–65 years, body mass index (BMI) 20–39 kg/m2, and generally good health. Exclusion criteria were tobacco use; acute or chronic inflammatory conditions; hypertension (blood pressure equal to or greater than 150 over 95 mmHg); liver or kidney dysfunction (self-reported or abnormal screening blood work); unwillingness to discontinue nutritional supplements (except for calcium, which was allowed at a stable dose); intake of fish, flaxseed, or walnuts equal to or greater than two or more servings per week; use of oral contraceptives or hormone replacement therapy; use of lipid-lowering, anti-inflammatory, anti-depressant, or blood pressure medication; and abnormal screening EKG or history of heart disease. Potential participants were advised that they would be expected to maintain low consumption of omega-3 fatty acids during the study, refrain from use of all supplements, and maintain their body weight.
A complete blood count and standard chemistry panel were obtained at screening to rule out the presence of serious illness (e.g., autoimmune disease, cancer, and immunodeficiency). Seated blood pressure was measured by nurses in a controlled environment using a calibrated mercury sphygmomanometer and appropriately sized cuffs, after a 5-min quiet rest according to JNC 7 guidelines [1]. Three readings were taken, and the average of the last two was used to determine eligibility for study participation and baseline characteristics. The blood pressure criterion (<150 mmHg systolic blood pressure [SBP] and <95 mmHg diastolic blood pressure [DBP]) was established to avoid excluding people with unmedicated stage 1 hypertension. The final study population was, on average, middle-aged (age mean = 44), overweight (body mass index mean = 29), and normotensive (mean BP = 123/82 mmHg). The sample was predominantly white, non-Hispanic, and contained one subject of Asian–Indian descent. The study was approved by the Pennsylvania State University Institution Review Board and registered on ClinicalTrials.gov (NCT00504309). Procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2000. All participants provided written informed consent.
Intervention
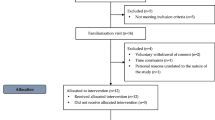
This was a randomized, double-blind, three-period crossover, placebo-controlled study with 8-week treatment periods and 6-week washout periods. Treatment was provided as four identical capsules per day during all periods. All capsules were provided by GlaxoSmithKline (Lovaza™ and identical corn oil placebo). Each 1 g prescription omega-3 fatty acid ethyl ester capsule contains approximately 465 mg EPA and 375 DHA (ratio of 1.2:1). During the three treatment periods, subjects received in random order: 0 g/day EPA + DHA (corn oil placebo), 0.85 g/day EPA + DHA, and 3.4 g/day EPA + DHA. The lower dose was provided as 1 g prescription omega-3 fatty acid ethyl esters and three placebo capsules. Treatments were matched to a coded numeric identifier so that the researchers and participants were blinded to treatment assignment. Subjects were instructed to maintain their weight and activity level during the course of the study, and they were counseled to exclude fatty fish meals (including salmon, tuna, mackerel, and herring), fish oil supplements, flax products, walnuts, and omega-3-enriched eggs during the study. Subjects were contacted 2 weeks into each phase to determine compliance and discuss any difficulties with taking the capsules. At the midpoint of each treatment period (4 weeks), subjects reported to the General Clinical Research Center to have their bottles weighed and to receive new supplies. Compliance was excellent (>95 % for all subjects during all periods) as determined by capsule logs and bottle weights. Erythrocyte EPA and DHA also increased in a dose-dependent manner in all subjects [23]. Body weight did not change during the study (data not shown).
Physiological Measures
Blood pressure was assessed with the Dinamap Pro 100 oscillometric monitor (GE Medical Systems) in the non-dominant arm. The appropriate cuff size was selected based on each participant’s arm circumference.
Systemic hemodynamic data were collected using thoracic impedance cardiography. Four band electrodes and three EKG electrodes were placed according to guidelines for impedance cardiography [24] as described previously [25]. Thoracic impedance was measured using a Hutcheson impedance cardiograph (HIC-2000, Instrumentation for Medicine, Greenwich, CT) and analyzed using the Cardiac Output Program (COP v5.08, Bio-impedance Technology, Inc., Chapel Hill, NC) [26]; cardiac output (liters per minute) = stroke volume × HR. Total peripheral resistance (TPR), also called systemic vascular resistance or afterload, was calculated from cardiac output and mean arterial pressure (MAP) (TPR in dyne s cm−5 = MAP × cardiac output/80) [27]. Pre-ejection period (duration of isovolumetric contraction), the Heather Index (a reflection of ejection velocity and contractility), and systolic time ratio (a measure of contractility that reflects the ratio of electrical to mechanical systole) were determined according to standard guidelines [28].
Tasks
During the 20-min resting baseline condition, participants listened to relaxing music.
The stressors employed in this study included a speaking task and a cold pressor task. The speech task required participants to mentally prepare (2-min preparation task) and then deliver a brief speech (3-min speech task) about a hypothetical situation. Each subject received one of the three different scenarios for each treatment endpoint testing session in random order. The scenarios described included: (1) the participant had been falsely accused of shoplifting by mall security, (2) the participant was pulled over for not stopping at an obscured stop sign, and (3) the participant was denied entry to an airline flight because they did not arrive 20 min prior to departure. Testing conditions were standardized by administering the speech instructions as a DVD recording.
Two experimenters observed the speech in an adjacent room via a video monitor. Participants were told that the experimenters were evaluating their poise, articulation, and appearance. Following the speech task, participants were monitored during a 10-min recovery period. Following the recovery period, DVD instructions were provided for the cold pressor task.
During the cold pressor task, participants were asked to immerse their foot up to the ankle in 4 °C water for 2.5 min. After this task participants recovered for a final 10-min period (cold recovery).
Testing Procedure
Cardiovascular tests occurred at the end of each treatment. Tests were scheduled in the afternoon and time of day was held constant within-subject. Four hours prior to testing, subjects were instructed to consume a light (low fat) meal and take half their daily capsule dose (two capsules). For the low-dose treatment, this dose included the active capsule. They were instructed to avoid pain relievers and alcohol (24 h), caffeine and decongestants (12 h), and exercise on the day of the visit. Compliance with pre-visit instructions was verified verbally. Subjects were rescheduled if they reported symptoms of acute infection.
BP, HR, and impedance measurements (averaged across intervals of 40 s) were obtained simultaneously during the testing session. SBP, DBP, and MAP were obtained oscillometrically. During the baseline resting period, measurements were obtained every 2 min to allow participants to adapt to the blood pressure cuff. Measurements were obtained every minute for the last 3 min of baseline and recovery periods. Measurements were obtained every minute during the preparation and stressor conditions.
The last three measurements of the resting and recovery periods were averaged. For the speech preparation, speech delivery, and cold pressor tests, measurements were taken every minute and averaged by task. These six task averages were treated as repeated measures for each testing session.
Determination of Erythrocyte Fatty Acids
At the beginning of the study and at the end of each treatment period blood samples were collected in the fasting state (12 h with nothing but water, 48 h without alcohol, and 2 h without vigorous exercise). Fasting blood samples were drawn into EDTA tubes. Erythrocytes were separated from plasma by centrifugation, and a 0.5-mL aliquot was collected from the RBC layer. RBCs were frozen at −80 °C until analyzed. RBCs were methylated to form fatty acid methyl esters which were analyzed by gas chromatography on a GC2010 (Shimadzu Corporation, Columbia, MD) equipped with a 100-m SP-2560 column (Supelco, Bellefonte, PA). The omega-3 index is the sum of EPA and DHA expressed as weight percent of total identified fatty acids [29].
Statistical Analysis
Statistical analyses were performed using SAS (Statistical Analysis System, Version 9.2, Cary, NC). Chi-square analysis was performed using the FREQ procedure. Normality was assessed prior to mixed models analysis. Only the Heather Index violated assumptions of normality, and a natural log transformation was applied prior to analysis. We report least squares means ± SEM. The mixed models procedure in SAS was used to test effects of treatment. This method has many advantages, including the fact that it allows users to model a variety of variance–covariance structures, its use of the correct error term in repeated measures, the ease of obtaining post hoc tests for designs with two repeated factors, and its robustness to occasional missing data [30]. Models included the following fixed effects: treatment, period (visit), task (e.g., baseline, speech, etc), and the treatment by task interaction. Subject was treated as a random effect. The treatment by period effect was universally nonsignificant and was removed from final models. When period effects were significant, they were retained. Post hoc tests were conducted with Tukey–Kramer adjusted p values (<0.05).
Regression was performed in Minitab (version 16.1.0, State College, PA). Outcomes with repeated measures were analyzed as an arithmetic mean (average across all tasks). Change scores were calculated as the end of treatment value minus end of placebo value. Residuals and residual vs. fit plots were examined to ensure homoscedascity. Regression slopes were judged to be equal by visual inspection (and by the absence of a significant treatment × predictor interaction), and therefore were calculated using pooled values (collapsed across the treatments).
Results
Participants
Twenty-six people completed the study (23 men and three postmenopausal women). The sample was predominantly white and non-Hispanic and included one subject of Asian–Indian descent. On average, participants were 44 years old with a BMI of 29.
Effects of Stressor Tasks
All stressor tasks (preparation, speech, and cold pressor) increased BP and HR compared to the resting condition (Table 1, Fig. 1). Specifically, the speech task increased MAP by 16 mmHg (main effect F(5,420) = 118.29, Tukey p < 0.0001, Cohen’s d = 1.40) and HR by 12 beats per minute (bpm, main effect F(5,422) = 69.85, Tukey p < 0.0001, Cohen’s d = 1.10). The cold pressor task produced similar increases in MAP but lesser increases in HR. HR returned to resting values during recovery periods, while BP remained slightly elevated relative to the resting condition. At the conclusion of the testing, participants completed a five-item post-task appraisal that assessed experiences of stress, coping, demand, threat, and performance; these results indicated consistent subjective responses to the tasks that did not differ by treatment or period (data not shown).Footnote 1
Omega-3 fatty acids significantly reduced heart rate, at rest and during stress. HR was dose-dependently different by treatment (p < 0.0001), and this pattern was evident throughout the rest and stressor periods. Relative to placebo, mean HR (collapsing across tasks) was reduced by 2.4 bpm following the low dose (p = 0.003) and 4.0 bpm following the high dose (p < 0.0001). Reductions in HR were greater with the high dose vs. low dose (p = 0.02). *Resting HR differed for the placebo and high dose by 4.0 bpm (p = 0.004)
Effects of Low and High Doses of Omega-3 Fatty Acids on Heart Rate (Fig. 1)
HR was dose-dependently different by treatment (Fig. 1); this was evident throughout the rest and stressor periods. The treatment effect was consistent, confirmed by an absence of a significant treatment by task interaction. Relative to placebo, mean HR collapsing across tasks was reduced by 2 bpm following the low dose (main effect F(2,422) = 21.12, Tukey p = 0.003, Cohen’s d = 0.23) and 4 bpm following the high dose (main effect F(2,422) = 21.12, Tukey p < 0.0001, Cohen’s d = 0.43). Reductions in HR were greater with the high dose vs. low dose (main effect F(2,422) = 21.12, Tukey p = 0.02, Cohen’s d = 0.24).
Resting HR following the high-dose treatment was 4 bpm lower than resting HR after placebo (main effect F(2,50) = 6.16, Tukey p = 0.004; Cohen’s d = 0.44; Fig. 1). The comparison of the low dose to placebo trended toward significance (main effect F(2,50) = 6.16, Tukey p = 0.07, Cohen’s d = 0.29).
Effects of Omega-3 Fatty Acids on Blood Pressure (Table 1)
MAP also differed by treatment with no treatment by task interaction (Table 1). The high dose reduced average MAP by about 2 mmHg (ΔSBP/DBP of 2.1/1.7 mmHg) relative to both the placebo (main effect F(2,420) = 5.39, Tukey p = 0.02, Cohen’s d = 0.23) and low-dose treatments (main effect F(2,420) = 5.39, Tukey p = 0.01, Cohen’s d = 0.23). The low dose did not alter BP. Analysis of resting values showed a similar pattern for treatment effects in that significant reductions in MAP were achieved after the high dose vs. the other two treatments. The high dose reduced resting MAP by 2.3 mmHg compared to placebo treatment (main effect F(2,50) = 4.44, Tukey p = 0.04; Cohen’s d = 0.30), and by 2.5 mmHg relative to the low-dose treatment (main effect F(2,50) = 4.44, Tukey p = 0.03; Cohen’s d = 0.33).
Thoracic Impedance Cardiography Measures (Table 2)
Due to electrical equipment failure not detected until post-study data analysis, results from impedance cardiography could be interpreted only for the first 13 participants. The characteristics of this subset of participants were similar to the full sample of participants (not shown). Treatment and task effects were significant for many measures that reflect autonomic tone (Table 2 and Electronic Supplementary Material Table 1). Compared to placebo, the high dose reduced stroke volume (main effect F(2,209) = 7.66, Tukey p = 0.003, Cohen’s d = 0.26) and cardiac output (main effect F(2,204) = 13.21, Tukey p < 0.0001, Cohen’s d = 0.39). The low dose reduced cardiac output (main effect F(2,204) = 13.21, Tukey p = 0.02, Cohen’s d = 0.20) compared to placebo, without reducing stroke volume. The high dose increased the pre-ejection period by 6.5 ms relative to placebo (main effect F(2,198) = 7.66, Tukey p = 0.0004, Cohen’s d = 0.36; Table 2). There was a shift to increased total peripheral resistance following omega-3 supplementation, but no post hoc pair-wise comparison reached significance.
Erythrocyte Fatty Acid Composition
As reported previously [23], erythrocyte EPA + DHA content (the omega-3 index) changed in a dose-dependent manner. At the end of the placebo treatment period, no subjects met the criterion for an optimal omega-3 index score (>8 % of total fatty acids [31]). On the high dose, 77 % achieved an omega-3 index >8 %, compared to one subject on the low dose who achieved that level (χ2[2] = 43.3, p < 0.0001).
Erythrocyte Fatty Acid Changes as Predictors of Cardiovascular Changes
Individual changes in the omega-3 index relative to placebo predicted average changes in MAP during reactivity sessions relative to placebo treatment (Fig. 2). The change in omega-3 index accounted for about 12 % of the variability in the change in MAP (standardized β [42] = −0.45, p = 0.02, r = 0.35); therefore, for every 1 percentage point increase in omega-3 index, MAP was reduced by approximately 1 mmHg. The slope of the regression line did not differ by treatment. There was no association between the changes in the omega-3 index and heart rate (data not shown).
Changes in the omega-3 index significantly predicted changes in blood pressure. Change in the omega-3 index (EPA + DHA of erythrocytes) relative to placebo treatment was used as a predictor of changes in mean arterial pressure during reactivity sessions relative to placebo treatment (Minitab v.16, State College, PA). The slope of the line did not differ by treatment and the two treatments were pooled for the analysis. The regression equation is ΔMAP = 2.03–1.07 Δomega-3 index
Discussion
Treatment with the high dose of omega-3 fatty acids (3.4 g EPA + DHA/day) significantly reduced HR and BP, and the magnitude of reduction did not differ between rest and stress conditions. In contrast, the low dose reduced HR relative to placebo, but had no effect on BP.
The reduction in BP following the high dose was driven by reductions in stroke volume and cardiac output. Although evidence suggests that total peripheral resistance could be decreased with omega-3 supplementation [32, 33], we observed a trend for increased resistance, which may reflect partial compensation for reductions in cardiac output. Results could differ if the effects of omega-3 fatty acids were measured under sustained physical stress [33, 34]; our measurements were taken under sedentary conditions. The significant increase in pre-ejection period may indicate that omega-3 fatty acids altered autonomic tone (increased vagal control of the heart with decreased adrenergic stimulation, in agreement with earlier studies [14–17]).
The magnitude of BP reduction with the high dose agrees with meta-analyses of responses to fish oil intervention [7–9]. In 31 placebo-controlled trials on 1,356 subjects, for every 1 g omega-3 fatty acids consumed, SBP/DBP decreased by 0.66/0.35 mmHg [7]. Thus, the predicted change in SBP/DBP on the high dose (−2.2/−1.2 mmHg) is similar to the change we observed (−2.1/−1.7 mmHg).
The reduction in HR we observed was greater than earlier meta-analyses would have predicted for our population and design, but is consistent with studies that used ambulatory monitoring [6, 35]. This consistency may be due to the increased statistical power of multiple measurements we used here and in ambulatory monitoring studies.
Regression analysis revealed that BP reductions were associated with changes in the omega-3 fatty acid content of erythrocytes. Since this is a relatively stable marker of intake that reflects changes in tissue levels, it could be speculated that BP effects are dependent on changes in tissue composition. Although we did not find a similar relationship with HR, an inverse relation between plasma DHA and HR has been reported [36]. In a recent study, serum DHA and EPA were inversely related to HR, and DHA additionally predicted resting and ambulatory DBP [37]. It may be that our sample size was too small and we were underpowered for this outcome.
The BP change that we observed would suggest that higher doses of EPA + DHA may potentially reduce risk of coronary events and stroke. It has been estimated that small BP reductions (around 5 mmHg) may prevent one-third of strokes and one fifth of coronary events in Western societies [38]. Although the 2 mmHg reduction for the high dose is modest, regression modeling suggests that deficient individuals (with an omega-3 index less than 4 %) given a high dose could achieve average BP reductions of 5 mmHg if their omega-3 index increases 6–7 %. Further, all subjects in this study had BP < 150/95 mmHg, and larger reductions might be achieved in people with Stage II hypertension (>160/100 mmHg) [39] or with longer-term treatment.
Omega-3 fatty acids are appealing as adjunctive therapy because they can safely be added to multidrug regimens and therapeutic lifestyle changes. The effects of omega-3 fatty acids on BP are additive to weight loss [40], potentiated by sodium restriction [41], and amplify the effects of beta-blockers [42] and diuretics [43]. Other than potential issues of cost and tolerability, there are few arguments against recommending high-dose omega-3 supplementation to patients as part of an overall treatment plan. It should be noted, however, that high-dose omega-3 fatty acid treatment is not currently indicated for lowering blood pressure; only for lowering triglycerides in patients with very high (>500 mg/dL) levels.
Despite the evidence for health benefits of omega-3 fatty acids, the average American diet lacks rich sources of omega-3 fatty acids, with estimated daily intakes <200 mg and omega-3 index (red blood cell EPA + DHA) values of 4–5 % [44].
There are limitations in our study. Our sample size was modest and consisted mainly of white males. Although we analyzed BP and HR data for all 26 participants, other hemodynamic data were only interpretable for half of these participants due to failure of the impedance cardiograph device. The duration of supplementation was only 8 weeks, which does not allow for a new membrane fatty acid steady state to be established [45]. Incorporating a 6-week washout between periods allowed 14 weeks separation between testing sessions; however, we cannot completely rule out the possibility that erythrocyte DHA exhibited some amount of carryover [23]. Another potential limitation is that cardiovascular testing was performed at the end of each period and not at study entry; however, treatment order was randomized and mixed modeling adjusted for habituation effects when they occurred. Stressors were presented in a fixed order and carryover effects may have been involved. It is important to note that we did not analyze reactivity scores (change from resting baseline) because omega-3 fatty acid supplementation had significant effects on resting measurements and no differential effects on the individual stress tasks. Therefore, while we cannot draw conclusions about the effects of omega-3 fatty acid supplementation on cardiovascular reactivity, our findings may be interpreted as evidence that omega-3 fatty acid supplementation affects systemic hemodynamics under conditions of rest and psychological/physiological stress. However, we may have been underpowered to detect significant treatment by task effects.
Conclusions
In summary, we demonstrated that omega-3 fatty acids have dose-dependent effects on BP, HR, and some aspects of systemic hemodynamics. Reductions in BP, stroke volume, and cardiac contractility were observed only with the higher dose, and the magnitude of BP reduction was predicted by increases in omega-3 fatty acids in erythrocytes. In contrast, HR was reduced by both doses in a dose–response manner and this change was not associated with changes in erythrocyte fatty acids. These findings support existing evidence that populations at risk for coronary artery disease with low omega-3 intake would achieve modest, dose-dependent benefits on BP and HR by adding oily fish and/or fish oil capsules to their diets.
Notes
The five response items were rated on 5-point Likert scale. Each response item was modeled using the mixed effects models applied to the other study outcomes and found to have universally nonsignificant effects for treatment, period, and treatment by period interaction.
References
Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560-2572.
Lang CC, Gupta S, Kalra P, et al. Elevated heart rate and cardiovascular outcomes in patients with coronary artery disease: Clinical evidence and pathophysiological mechanisms. Atherosclerosis. 2010;212:1-8.
Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973-1983.
Investigators G-P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447-455.
Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet. 1989;2:757-761.
Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: A meta-analysis of randomized controlled trials. Circulation. 2005;112:1945-1952.
Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523-533.
Appel LJ, Miller ER 3rd. Seidler AJ, Whelton PK: Does supplementation of diet with 'fish oil' reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429-1438.
Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J Hypertens. 2002;20:1493-1499.
Bonaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromso study. N Engl J Med. 1990;322:795-801.
Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T. Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med. 1995;123:911-918.
Grundt H, Nilsen DW, Hetland O, et al. Improvement of serum lipids and blood pressure during intervention with n-3 fatty acids was not associated with changes in insulin levels in subjects with combined hyperlipidaemia. J Intern Med. 1995;237:249-259.
Vandongen R, Mori TA, Burke V, Beilin LJ, Morris J, Ritchie J. Effects on blood pressure of omega 3 fats in subjects at increased risk of cardiovascular disease. Hypertension. 1993;22:371-379.
Hamazaki K, Itomura M, Huan M, et al. Effect of omega-3 fatty acid-containing phospholipids on blood catecholamine concentrations in healthy volunteers: A randomized, placebo-controlled, double-blind trial. Nutrition. 2005;21:705-710.
Hamazaki T, Sawazaki S, Nagasawa T, Nagao Y, Kanagawa Y, Yazawa K. Administration of docosahexaenoic acid influences behavior and plasma catecholamine levels at times of psychological stress. Lipids. 1999;34:S33-S37.
Sawazaki S, Hamazaki T, Yazawa K, Kobayashi M. The effect of docosahexaenoic acid on plasma catecholamine concentrations and glucose tolerance during long-lasting psychological stress: A double-blind placebo-controlled study. J Nutr Sci Vitaminol (Tokyo). 1999;45:655-665.
Delarue J, Matzinger O, Binnert C, Schneiter P, Chiolero R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289-295.
Christensen JH. n-3 fatty acids and the risk of sudden cardiac death. Emphasis on heart rate variability. Dan Med Bull. 2003;50:347-367.
Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med (Hagerstown). 2007;8(Suppl 1):S19-S22.
Ginty AT, Conklin SM. Preliminary evidence that acute long-chain omega-3 supplementation reduces cardiovascular reactivity to mental stress: A randomized and placebo controlled trial. Biol Psychol. 2012;89:269-272.
West SG, Krick AL, Klein LC, et al. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr. 2010;29:595-603.
Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747-2757.
Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243-252.
Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1-23.
West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135:212-217.
McFetridge J, Sherwood A. Impedance cardiography for noninvasive measurement of cardiovascular hemodynamics. Nurs Res. 1999;48:109-113.
Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJP. Committee report: Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1-23. http://www.sprweb.org/articles/Sherwood90.pdf.
McFetridge J, Sherwood A. Impedance cardiography for noninvasive measurement of cardiovascular hemodynamics. Nurs Res. 1999;48:109-113.
Harris WS, Von Schacky C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev Med. 2004;39:212-220.
Bagiella E, Sloan RP, Heitjan DF. Mixed-effects models in psychophysiology. Psychophysiology. 2000;37:13-20.
Harris WS. The omega-3 index: From biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411-417.
Mozaffarian D. Fish, n-3 fatty acids, and cardiovascular haemodynamics. J Cardiovasc Med (Hagerstown). 2007;8(Suppl 1):S23-S26.
Walser B, Stebbins CL. Omega-3 fatty acid supplementation enhances stroke volume and cardiac output during dynamic exercise. Eur J Appl Physiol. 2008;104:455-461.
Monahan KD, Wilson TE, Ray CA. Omega-3 fatty acid supplementation augments sympathetic nerve activity responses to physiological stressors in humans. Hypertension. 2004;44:732-738.
Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253-260.
Grimsgaard S, Bonaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68:52-59.
Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-Chain Omega-3 Fatty Acids and Blood Pressure. Am J Hypertens. 2011;24:1121-1126.
MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765-774.
Mori TA. Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol. 2006;33:842-846.
Bao DQ, Mori TA, Burke V, Puddey IB, Beilin LJ. Effects of dietary fish and weight reduction on ambulatory blood pressure in overweight hypertensives. Hypertension. 1998;32:710-717.
Cobiac L, Nestel PJ, Wing LM, Howe PR. A low-sodium diet supplemented with fish oil lowers blood pressure in the elderly. J Hypertens. 1992;10:87-92.
Singer P, Melzer S, Goschel M, Augustin S. Fish oil amplifies the effect of propranolol in mild essential hypertension. Hypertension. 1990;16:682-691.
Lungershausen YK, Abbey M, Nestel PJ, Howe PR. Reduction of blood pressure and plasma triglycerides by omega-3 fatty acids in treated hypertensives. J Hypertens. 1994;12:1041-1045.
Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155-160.
Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J Lipid Res. 1997;38:2012-2022.
Acknowledgments
This study was funded by a scholarship grant from the National Fisheries Institute. Study materials (capsules) and additional financial support was provided by Reliant Pharmaceuticals (now GlaxoSmithKline, GSK). Financial supporters had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. We are grateful to Danette L. Teeter for her analysis of the impedance cardiography results. Katherine A. Sauder provided valuable assistance with revision of the manuscript. We are also grateful to the nursing and clinician staff of the General Clinical Research Center of The Pennsylvania State University, which was supported by NIH Grant M01 RR 10732.
Conflict of Interest Statement
WSH is a scientific adviser to companies with interests in fatty acids including Omthera, Aker Biomarine, and GlaxoSmithKline, and has been a speaker for the latter. In addition, he is the owner of OmegaQuant Analytics, LLC (Sioux Falls, SD) and a Senior Scientist at Health Diagnostic Laboratory (Richmond, VA); both are companies that offer blood omega-3 fatty testing. The other authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 25 kb)
About this article
Cite this article
Skulas-Ray, A.C., Kris-Etherton, P.M., Harris, W.S. et al. Effects of Marine-Derived Omega-3 Fatty Acids on Systemic Hemodynamics at Rest and During Stress: a Dose–Response Study. ann. behav. med. 44, 301–308 (2012). https://doi.org/10.1007/s12160-012-9393-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-012-9393-2