Abstract
Background
Research in the past 20 years has shown that self-assessed health (SAH) is a consistent and reliable predictor of health outcomes. However, it is still unclear what factors are responsible for the association of SAH with mortality and other objective health indicators.
Purpose
This study examined the impact of trait positive affect, trait negative affect, and functional limitations (FL) on SAH cross-sectionally and longitudinally. We hypothesized that changes in SAH ratings would be mainly influenced by FL, whereas affective information would have a biasing effect on SAH ratings cross-sectionally.
Methods
We analyzed longitudinal data obtained from five successive annual interviews conducted with over 800 elderly respondents (mean age = 73 years) using latent growth curve modeling.
Results
Results revealed that SAH judgments were related to FL and to trait affects in each of the five waves (cross-sectional data), but only changes in FL were related to changes in SAH over time. These data also showed that changes in SAH were predictive of mortality above and beyond its initial levels.
Conclusions
The results suggest that the temporal trajectory of FL is a source of information that allows an individual’s SAH to predict objective health measures of both morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robust relationships of self-assessments of health (i.e., “How would you rate your health?”) with mortality and morbidity have been reported in over 200 studies conducted in different national settings and diverse groups of clinically ill patients [1, 2]. Unlike other self-appraisals [3], the association of self-assessments of health (SAH) with objective outcomes such as mortality and morbidity is maintained after adjusting for objective health indicators (e.g., blood pressure, smoking status, and co-morbid conditions). Additionally, evidence has shown that SAH ratings are sensitive to changes in individuals’ physical health [4] and that these changes predict mortality [5, 6]. The strength and consistency of these findings has motivated a search for the information people use in making these self assessments [7]. Affective traits and states, both negative and positive, have been prime candidates [8, 9] as have been factors such as the individual’s functional status, symptoms, and illness history [10–12]. Although all of the aforementioned factors have been associated with SAH ratings, the data have yet to provide a clear answer as to which, among these factors, are critical for updating SAH judgments over time and, most importantly, for the relationship of SAH to objective indicators such as mortality. We propose that SAH is a dynamic factor whose association with mortality is significant because individuals rate their own health by relying on a limited set of dynamic, concrete illness-related aspects such as functional limitations (FL) [13].
The current study examined multi-wave data from a longitudinal study of over 800 community dwelling elderly respondents to assess how FL and affective factors [i.e., trait positive affectivity (PA) and trait negative affectivity (NA)] relate to SAH ratings. Specifically, we assessed whether these factors have only a cross-sectional association with SAH as shown in prior studies or whether they are also important for updating SAH judgments longitudinally. The hypothesis underlying our analyses was that both the stable and state-like features of affective traits would yield substantial relationships of trait NA and trait PA to SAH in cross-sectional analyses but that these variables would not relate with changes in SAH over time. These cross-sectional or concurrent relationships were posited to reflect what has been termed “hot” decision making [14, 15].
The consistent association of SAH with mortality, an objective health outcome, suggests that SAH must reflect valid, context-independent input in addition to temporary influences [16]. If we assume that trait PA and trait NA assess an individual’s readiness to experience positive and/or negative moods [17], we can expect that individuals with high levels on these traits will be more likely to experience fluctuations in mood that will increase access to mood congruent information [18]. Thus, individuals who score high on PA should be more likely to make favorable SAH judgments, and individuals scoring high on trait NA should be more likely to make less favorable SAH judgments at any given point in time. The fluctuation in mood states will dilute the contribution of trait NA and trait PA to changes in SAH over time. Available data are consistent with the assumption that associations of trait NA and trait PA to SAH judgments will be found in cross-sectional data, given that the measures of affective variables contain both trait and state components. For example, significant and independent negative correlations of SAH to measures of trait NA and positive correlations to measures of trait PA are found in analyses of data collected in community settings [9, 10]. Similar cross-sectional relationships between SAH and positive and negative moods have been observed in field studies [19]. In settings where moods can be attributed to the immediate context (i.e., experimentally induced-moods), evidence shows that trait NA but not experimentally induced moods influence SAH ratings [8].
The commonsense model of illness cognition (CSM) [20] provides a framework for clarifying the role that trait affectivity and functional limitations play in the elaboration of self-assessments of health. The CSM postulates that people are problem solvers who actively assess somatic changes and form hypotheses about their potential meaning. They attribute meaning to or label their somatic sensations based on their pattern (identity), their awareness of exposure to viruses, stress, or others who are ill (cause); the duration of the symptoms (timeline); their ability to respond to the health threat (control); and the impact on their current function (consequences). The commonsense “self-diagnosis” an individual assigns to his or her somatic changes or functionality problems is a product of the match between this active appraisal process and underlying prototypes or schemata of various acute and/or chronic illnesses [21]. These prototypes influence attention to the body and the somatic events dominant in verbal reports, and they shape the selection and evaluation of strategies to regulate the presumed, underlying condition.
A similar process is assumed to generate prototypes or schemata of the physical and psychological self. We posit that physical and psychological function are checked against similar prototypes, sometimes explicitly, when judging current “health” and updating the representation of the self [22]. Over time, individuals will develop a degree of expertise in judging when they are sick or well by checking their psychological and physical experiences against underlying schemata of illness and the self. This expertise allows them to appraise whether a somatic or functional change has significance with respect to health, and if so, whether it has acute or short-term significance or implies a change that is chronic and has long-term significance for health. Specifically, elderly people can quickly recognize and categorize physical dysfunction due to an accident such as a bone fracture caused by a falling object, in contrast to a broken bone caused by a fall due to increasing unsteadiness when walking. These schemas allow for the sorting of information as health or non-health relevant, which, in turn, is reflected in SAH ratings [13, 23]. The proposition that people rely on concrete health-related information when assessing their health status led us to propose that FL are a critical factor in this process. Experiencing increases in FL over a long duration (timeline) in the absence of attribution to a temporary cause (e.g., slipping on a wet floor) can set the stage for the revision of self-schemata as having a condition that will have a permanent impact on one’s daily life [24] and elicit depressive affect [25]. Existent data support the hypothesis of a strong association between FL and SAH, FL having a greater impact on SAH ratings than indicators such as medical history and recent chronic conditions [26].
Our specific expectations were as follows:
-
1.
As health and functional status decline with age, we expected that SAH judgments of our elderly participants would decline over the 5-year period of our data collection. Additionally, we hypothesized that if these declines in SAH reflect valid health information, then these changes would predict mortality above and beyond initial levels of SAH.
-
2.
We expected that if FL were a critical factor for SAH’s predictive validity, then ratings of limitations would increase at a rate similar to SAH over the time period of this study. Consistent with other data, trait PA and trait NA were expected to remain relatively stable over time.
-
3.
Based on the idea that factors that bias SAH ratings would operate only concurrently while factors that provide valid information would operate both concurrently and over time, we hypothesized that: (a) trait PA and trait NA would be associated with SAH judgments in cross-sectional but not longitudinal analyses (i.e., these traits would not influence changes in SAH over time) and (b) FL would be associated with SAH judgments in both cross-sectional and longitudinal analyses, i.e., increases in FL over 5 years would relate to declines in SAH over this time period.
Method
Participants
Data for these analyses were obtained during the first five waves (1991–1995) of a 9-year longitudinal survey of community-dwelling older adults in central New Jersey. A total of 851 participants provided data at baseline, 791 at wave two, 719 at wave three, 678 at wave four, and 554 at wave five. Subject loss averaged 9.75% per year due, in approximately equal parts, to death, moving away from the community, and withdrawal from the study. The mean age at baseline was 73 years (range = 49–93), and 83.6% of the participants were over 65 years of age. The sex division at baseline was 479 women (56%) and 311 men (44%), and 72% of women and 80% of men had more than 12 years of education.
Design and Procedure
The design of the larger study was both cross-sectional and longitudinal, and its main focus was the investigation of the relationships between emotions and indicators of physical health. The data were collected in a series of annual, in-depth, in-person interviews. Brief telephone follow-up interviews were conducted between each of these annual interviews. Interviewers recorded participants’ responses on laptop computers using CASES software, a computer-assisted survey methodology [27]. Interviews averaged approximately 2 h in length, and telephone follow-ups lasted approximately 40 min. A more detailed description of subject recruitment and procedures can be found in Benyamini et al. [12].
We analyzed the first five waves of data because all of the measures of interest were assessed for each of those years. After the fifth year, the interview was modified, and several of the measures used in the present study were assessed at irregular intervals or not at all.
Main Measures
Self-Assessed Health
The interview opened with a single question asking participants to make an overall assessment of their health: “In general, how would you rate your health…5 = excellent, 4 = very good, 3 = good, 2 = fair, or 1 = poor?” Interviewers reported that, on average, participants took only a few seconds to respond to this question.
Functional Limitations
Limitations in daily function were assessed with six items: Does your health limit the kinds or amounts of (a) vigorous activities you can do such as running, lifting heavy objects, or participating in strenuous sports or activities?; (b) moderate activities you can do such as moving a table, carrying groceries, bending, or lifting?; do you have any trouble (c) walking one block, uphill, or a few flights of stairs?; (d) eating, dressing, bathing, or using the toilet?; and how much do problems with your health stand in the way of doing the things (e) you would like to do and (f) you need to do” (the response scale ranged from 1 = not at all to 5 = very much). The final score for each subject was computed by averaging each participant’s responses to these six items. The internal consistency (Cronbach’s alpha) for this scale over the 5 years ranged between 0.81 and 0.90.
Trait Negative Affect
Negative affect was assessed with two five-item adjective lists, one for depressed (i.e., sad, blue, glum, gloomy, and depressed) and one for anxious (i.e., tense, nervous, uneasy, worried, and on edge) mood. Each question asked, “How ______ are you usually?,” and responses were recorded on 5-point Likert scales (1 = not at all to 5 = very). A negative affect score was computed by averaging the responses to the ten items. The internal consistency (Cronbach’s alpha) for the scale over the 5 years ranged between 0.92 and 0.93.
Trait Positive Affect
Positive affect was assessed with a five-item adjective list (i.e., pleased, content, happy, cheerful, and enthusiastic). Each question asked, “How ______ are you usually?,” and responses were recorded on 5-point Likert scales (1 = not at all to 5 = very). The positive affect score was computed by averaging the responses to the five items. The internal consistency for the scale over the 5-year period ranged between 0.85 and 0.89.
Statistical Analyses
Analyses were conducted in four steps. First, separate univariate models assessed stability and/or change for SAH, trait PA, trait NA, and FL. These models tested whether a given factor followed our expectations and fit a dynamic model or a trait model. Univariate models compared the fit to the data of latent growth curve models with or without autoregressive effects (autoregressive latent trajectory models or ALT) [28]. Each model examined whether the inclusion of one or two latent factors better represented the data: (a) an intercept that captured the average value of the variable at the start of the process and/or (b) a growth factor that represented a linear change over time (i.e., slope). We also tested whether it was tenable to include autoregressive parameters in the model (i.e., each observed measure in a given wave was regressed on the same measure assessed in the prior wave). To prevent misspecification, the baseline measure in each model was treated as a predetermined or exogenous variable and allowed to co-vary freely with both latent factors.
We next estimated bivariate models that examined the respective relationship of trait PA, trait NA, and FL with SAH over the 5-year period (cf. Figs. 1 and 2). These analyses examined the unique impact of each of the predictors on SAH. Each bivariate model maintained the parameters from the univariate models and added paths to examine the associations between each set of constructs. A multivariate model, computed at step 3, examined the simultaneous effects of trait PA, trait NA, and FL on SAH. This model was specified based on the parameters from both univariate and bivariate models. The multivariate model assessed whether the inclusion of multiple predictors altered the concurrent effects and the associations among latent and observed variables. A final analysis used this multivariate model to assess the impact of SAH on mortality. This analysis modeled participant’s survival as a proportional hazard dependent upon individual differences in time 1 SAH and SAH intercept and upon changes in SAH over time (details of the mathematical model can be found in [29]).
All analyses were conducted using Mplus version 5.0. ALT and latent growth models were estimated using full information maximum likelihood (FIML) estimation under the assumption of data missing at random (MAR) [30] with robust standard errors (MLR estimator in Mplus) [31]. MAR assumes that the probabilities of values being missing can be predicted from variables that are not missing such as variables assessed at the first wave [32]. In longitudinal studies such as the current one, Y scores for missing cases show a MAR pattern as they are correlated with their own scores from earlier waves. This estimation procedure yields less biased parameter estimates than the analyses restricted only to those cases with complete data even when MAR assumptions are not met [31].
Model fit was evaluated using a rescaled χ 2 test (i.e., the T2* statistic) [31], the comparative fit index (CFI), the Tucker–Lewis fit index (TLI), the root mean squared error of approximation (RMSEA), and the standardized root mean square residual (SRMR). Comparison of nested models was conducted with the procedure described by Satorra and Bentler [33].
Results
Descriptive Results
Table 1 shows the descriptive statistics of SAH, trait PA, trait NA, and FL and the between-wave correlations. Declines in SAH from times 1–5 indicated that our elderly participants reported poorer health with the passage of time. FL also increased over time with the increase decelerating at time 5. Means for both trait NA and trait PA were relatively stable over time, showing no clear growth patterns.
The between-wave correlations were typical for associations among repeated measures; observations taken closer in time are more strongly correlated with one another than observations taken further apart. This pattern of associations indicates substantial individual differences in change over time.
Univariate Models
As expected the model specifying decreases in SAH over time had an excellent fit to the data (cf. Table 2). The inclusion of autoregressive effects did not improve the fit of the model [\(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{4}} \right) = {\text{4}}{\text{.40}}\), p = 0.35]. Furthermore, none of the autoregressive effects differed from zero (β’s ranged between −0.05 and 0.09, z’s = −0.57–1.20, p’s ≥ 0.23). Intercept-only models with or without autoregressive effects were not supported by the data. The value of the intercept at time 2 was M i = 3.35, z = 143.72, p < 0.01 (variance was V i = 0.58, z = 14.90, p < 0.01). The slope of the latent factor showed that SAH ratings decreased an average of 0.07 units or about 2.1% (effect size r = 0.20) each year (z = −5.84, p < 0.01; V s = 0.03, z = 4.67, p < 0.01). The intercept and slope were uncorrelated (r is = −0.11, z = −1.20, p = 0.23). The significant variances for both latent factors highlighted important individual differences in both intercept and slope. Although the proportion of variance explained in each of the dependent variables (i.e., time 2 to time 5 SAH) was high (r 2s = 0.66–0.78), all residual variances were significantly different from zero (Ψs = 0.22–0.30, zs ≥ 6.14, ps < 0.01), indicating that changes over time did not fully account for all the variability in observed SAH measures.
Increased in FL over time also supported our expectations. A model specifying a linear change without autoregressive effects and an unconstrained loading for the final wave had an excellent fit to the data (see Table 2). Both the mean intercept (M i = 1.73, z = 101.36, p < 0.01; V i = 0.49, z = 17.38, p < 0.01) and the mean slope (M s = 0.10, z = 8.77, p < 0.01; V s = 0.03, z = 3.49, p < 0.01) were statistically different from zero. On average, FL increased about 6% a year (effect size r = 0.28). The inclusion of autoregressive effects did not improve model fit, \(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{4}} \right) = {\text{6}}{\text{.05}}\), p = 0.20, and the data did not support an intercept only model with or without autoregressive effects; intercept and slope were uncorrelated (r is = −0.16, z = 1.51, p = 0.13).
Analyses showing that trait PA did not change over time provided support for our expectations. An ALT model with an intercept factor alone fit the data well (cf. Table 2). The mean intercept value for this model was M i = 3.07, z = 20.32, p < 0.01 (variance was V i = .21, z = 8.81, p < 0.01), and all autoregressive effects were significant (βs ranged between 0.16 and 0.18, z values ≥3.90, all p’s < 0.01) indicating that inter individual differences in trait PA are also stable over time. Imposing equality constraints on the autoregressive effects or constraining autoregressive effects to zero did not improve model fit.
As hypothesized, the data revealed that individual differences in trait NA were also stable over time. An intercept-only model with autoregressive effects and a correlated error between the second and third waves had an excellent fit to data (see Table 2). All the autoregressive effects were significant: β’s ranged between 0.14 and 0.24, z values ≥2.93, p’s < 0.01. The mean intercept value for this ALT model was M i = 1.31, z = 19.16, p < 0.01 (variance was V i = 0.16, z = 8.36, p < 0.01). Imposing equality constraints on the autoregressive effects or constraining autoregressive effects to zero resulted in models with very poor fit to data.
Bivariate Models
Self-Assessed Health and Trait Positive Affectivity
To test the hypothesis that trait PA would be associated with SAH cross-sectionally but not longitudinally, we combined their univariate models into a single, associative one (see Fig. 1). This model specified concurrent effects of observed PA on observed SAH (i.e., time 2 PA predicting time 2 SAH, time 3 PA predicting time 3 SAH, etc.); these effects were constrained to be equal across waves. In addition, the following covariances were constrained to be 0: (1) slope of SAH with time 1 PA and (2) slope of SAH with intercept of PA.
Bivariate model testing the association between trait affect and SAH over time. Double-headed arrows indicate freely estimated covariances. Dashed lines indicate cross-lagged effects that were not included in the hypothesized model. Covariance between trait affect and SAH intercept was omitted for sake of clarity. Error terms are not shown
The fit to the data of the hypothesized model was excellent (see Table 2). Concurrent effects of PA on SAH were significant (cf. Table 3); high levels of PA were associated with high SAH scores at the same wave. The correlation between the intercepts of trait PA and SAH was also positive and significant (r = 0.46, z = 9.73, p < 0.01). The addition of cross-lagged effects from PA to SAH (e.g., time 1 PA to time 2 SAH, etc.) and from SAH to PA (i.e., time 1 SAH to time 2 PA, etc.) did not improve model fit, \(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{8}} \right) = {\text{12}}{\text{.37}}\), p = 0.14. SAH residual variances were all significantly different from zero (Ψ’s ranged between 0.23 and 0.30, z’s ≥ 6.33, p’s < 0.01).
Self-Assessed Health and Trait Negative Affectivity
The constraints for the model examining the association between SAH and trait NA were similar to those described above for trait PA. Although this model had an adequate close fit to the data, it is one that could be improved upon (see Table 2). The concurrent paths from NA to SAH were all significant, β’s = −0.08–0.09, z’s < −3.31, all p’s < 0.01 (see Table 3), indicating that trait NA has a modest impact on SAH ratings cross-sectionally. Significant residual variances (Ψ’s = 0.23–0.34, z’s ≥ 6.68, p’s ≤ 0.01) indicated that this model did not account for all the variability in observed SAH measures.
Neither the free estimation of the covariances set to zero [\(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{2}} \right) = {\text{3}}{\text{.88}}\), p = 0.14] nor the removal of the equality constraint of the concurrent paths from NA to SAH [\(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{3}} \right) = {\text{4}}{\text{.11}}\), p = 0.25] improved the model’s fit. The addition of cross-lagged effects was not supported by the data [\(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{8}} \right) = {\text{12}}{\text{.37}}\), p = 0.14]; all but one cross-lagged effect were non-significant. Fit diagnostics for the hypothesized model did not suggest any theoretically relevant change; therefore, the hypothesized model was retained.
Self-Assessed Health and Functional Limitations
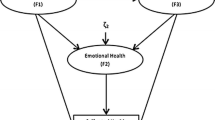
This bivariate model tested the hypothesis that FL would influence SAH ratings cross-sectionally and longitudinally (see Fig. 2). This model freely estimated the following paths: (1) concurrent effect of FL on SAH (e.g., time 2 FL to time 2 SAH; etc.), which were constrained to be equal across waves; (2) SAH intercept was regressed on FL intercept, and its residuals were allowed to covary with time 1 SAH; (3) SAH slope was regressed on the intercept and the slope of FL; and (4) time 1 SAH, time 1 FL, and the intercept and the slope of FL were allowed to freely covary. In addition, based on the results of the univariate models, autoregressive effects for both SAH and FL were constrained to zero.
Bivariate model testing the association between functional limitations and SAH over time. Double-headed arrows indicate freely estimated covariances. Dashed lines indicate cross-lagged effects that were not included in the hypothesized model. Dotted lines indicate regression paths from FL intercept and slope to SAH slope. Regression path from FL intercept to SAH intercept was omitted for sake of clarity. Error terms are not shown
Table 2 shows that the fit of this model to the data was excellent. All concurrent paths from FL to SAH were significant at all time points with high levels in FL associated with low levels of SAH (see Table 3), and changes in SAH (i.e., SAH slope) were significantly predicted by changes in FL (β = −0.44, z = −4.41, p < 0.01) but not by the intercept of functional limitations (β = 0.12, z = 1.13, p = 0.26). SAH intercept was significantly predicted by the intercept of FL (β = −0.54, z = −8.89, p < 0.01). Correlations among intercept of SAH, time 1 FL, and FL intercept were significant (r’s ranged between |0.51| and |0.87|, z’s ≥ |11.86|, p’s < 0.01. Changes in FL (i.e., slope) were not associated with the intercept of FL (r = 0.03, z = 0.19, p = 0.85), time 1 FL (r = 0.13, z = 1.48, p = 0.14), or time 1 SAH (r = −0.13, z = 1.80, p = 0.07). As in the previous bivariate models, significant residuals remained at each wave of SAH (Ψ’s = 0.22–0.30, z’s = 6.78–13.33, p’s < 0.01). Overall, the results revealed that FL played a critical role in both determining participants’ SAH judgments at each wave and influencing their trajectory over time.
The model estimating cross-lagged effects also had an adequate fit and was significantly better than the constrained model [\(\chi _{{\text{diff}}}^{\text{2}} \left( {\text{8}} \right) = {\text{21}}{\text{.30}}\), p < 0.01]. However, the cross-lagged effects were not statistically different from zero (paths from FL to SAH, β’s = 0.05 to.12, z’s = 0.86–1.67, p’s > 0.09; paths from SAH to FL, β’s = −0.04 to −0.05, z’s = −1.50 to −1.30, p > 0.13). Accordingly, we retained the more parsimonious and hypothesized model. Comparisons of information criteria values [34] supported this decision (AICdiff = 4.77, BICdiff = −33.02, and sample-size adjusted BICdiff = −7.80).
Multivariate Model: Simultaneous Effects of Positive and Negative Affectivities and Functional Limitations on Self-Assessed Health
All univariate models were combined to conduct a multivariate model that tested the simultaneous influence of trait PA, trait NA, and FL on SAH. This model estimated the concurrent effects of observed trait PA, trait NA, and FL on observed SAH (e.g., time 2 trait NA, trait PA, and FL predicting time 2 SAH, time 3 trait NA, trait PA, and FL predicting time 3 SAH, etc.). Each set of associations was constrained to be equal across the four waves. Based on the previous results, changes in SAH were predicted only by changes in FL. Additionally, the intercept of SAH was predicted by the intercept of trait PA, trait NA, and FL. We allowed the residuals of the SAH intercept to covary with time 1 SAH. Cross-lagged effects for trait PA and trait NA were freely estimated, and each set was constrained to be equal. In addition, the covariances between FL slope with trait PA intercept, trait NA intercept, time 1 trait PA, time 1 trait NA, and time 1 SAH, respectively, were constrained to zero. All other covariances among exogenous variables were freely estimated. In addition, the within-time residuals among predictor measures (i.e., trait PA, trait NA, and FL) were allowed to covary freely.
Results showed an adequate fit to data (cf. Table 2). Observed SAH ratings were significantly predicted by concurrent observed FL and PA but not by NA (see Table 3). It is important to note, however, that the strength of association of NA to SAH was similar to that of PA to SAH. Changes in SAH were significantly predicted by changes in FL (β = −0.35, z = −2.48, p < 0.05). The intercept for SAH was predicted by the intercept of PA (β = 0.27, z = 4.83, p < 0.01), NA (β = −0.14, z = −2.63, p < 0.01), and FL (β = −0.42, z = −7.38, p < 0.01). The residuals of the intercept of SAH were significantly correlated with time 1 SAH (r = 0.53, z = 16.57, p < 0.01). These results also showed that all the correlations among observed and latent exogenous variables were statistically significant (see Table 4). All autoregressive and cross-lagged effects for trait PA (autoregressive: β’s = 0.12 to 0.14, z’s ≥ 3.20, p’s < 0.01; cross-lagged trait NA to trait PA: β’s = −0.12 to −0.13, z’s ≤ −4.12, p’s < 0.01) and trait NA (autoregressive: β’s = 0.11 to 0.21, z’s ≥ 2.53, p’s < 0.05; cross-lagged trait PA to trait NA: β’s = −0.08 and −0.09, z’s ≤ −2.79, p’s < 0.01) were statistically significant. This model accounted for a substantial proportion of the variance in observed and latent SAH variables (range from 0.66 to 0.76 for observed variables; intercept, R 2 = 0.42; slope, R 2 = 0.12). The results also indicated that residual variances were significant for observed measures of SAH (Ψ’s = 0.23–0.30, z’s = 7.26–13.23, p’s < 0.01) and for the intercept and slope of SAH (intercept Ψ = 0.21, z = 12.27, p < 0.01; slope Ψ = 0.02, z = 4.47, p < 0.01).
Multivariate Model Predicting Mortality: Effects of the Intercept and Slope of Self-Assessed Health on Mortality
The final analysis examined whether changes in SAH predicted mortality above and beyond the intercept of SAH. To conduct this analysis, we used the multivariate model described above as the base model and added mortality as an outcome. The model specified time-to-death as a function of only time 1 SAH, SAH intercept, and SAH slope. The results showed that each unit decrease in the intercept of SAH was associated with a 72% increase in risk of mortality [hazard ratio (HR) = 0.38, 95% confidence interval (CI) = 0.17, 0.85] and that each unit decrease in the slope of SAH was associated with a 98% increase in the risk of mortality (HR = 0.02, 95% CI = 0.001, 0.55). In other words, for the average person whose SAH ratings decreased 2% during a year, the risk of dying increased by roughly 57%. Time 1 SAH was not associated with mortality (HR = 0.99, 95% CI = 0.64, 1.52).
Discussion
The modeling strategy used in this study allowed us to simultaneously test the cross-sectional and longitudinal effects of affective traits and functional limitations on SAH judgments. By using five consecutive years of data, we were able to test the hypothesis that trait PA and trait NA would relate to SAH judgments cross-sectionally (i.e., a biasing effect) while FL would have a systematic influence in both cross-sectional and longitudinal analyses.
Univariate analyses showed a significant decline in SAH over the 5 years of data. The absence of significant autoregressive effects for SAH extended previous research, demonstrating that individual differences in SAH are not consistent over time; participants’ SAH ratings started at different points and changed at different rates consistent with evidence that individual health trajectories differ over time [35]. The increase in FL over the 5-year period is consistent with evidence showing that people experience an increasing number of health problems as they age. The significant variability in changes in FL agrees with findings showing that FL increase at different rates for different individuals [24]. The data are at variance, therefore, with treating reports of functional limitations as a trait. In contrast, and as expected, the univariate models showed that trait PA and trait NA were stable. The significant autoregressive effects for both trait PA and NA indicate that a substantial amount of the variance in these measures reflects a tendency for individuals to experience and report similar levels of positive and negative affect over time [36].
Bivariate models supported the hypothesis that affective experiences would be associated with SAH cross-sectionally but not longitudinally. Both trait PA and trait NA had modest associations with SAH when rated concurrently, and these associations were stable over time. The absence of an association of changes in SAH with the intercepts of either trait PA or trait NA further supported our hypothesis. The results for the association between FL and SAH, on the other hand, showed associations between these factors both concurrently and over time (i.e., their respective trajectories or slopes were associated). Thus, decreases in SAH were related only to increases in functional limitations over the 5-year time frame studied. The association between changes in both SAH and FL suggests that the decline in SAH ratings is due partly to increases in functional limitations. Together with the lack of support for cross-lagged effects, these results strongly suggest that current, not past health problems are the main source of information that people use to appraise and rate their health. The multivariate model strengthened the support for the hypothesis that changes in function play a critical role in informing SAH ratings. When all three measures (i.e., FL, trait PA, and trait NA) were entered, the concurrent association of FL with SAH was unchanged, but the magnitude of association of the trait NA and PA with SAH was reduced. This multivariate model also confirmed that only changes in FL predicted changes in SAH.
The data indicate that a dynamic self-appraisal process underlies SAH ratings made by our elderly participants. To determine that the contribution of a particular factor to this process enhances the predictive validity of SAH ratings rather than function as a temporary source of bias, the variable must be related to SAH judgments concurrently [37] and have a trajectory similar in pattern to that of SAH over time. In contrast, a biasing factor would only relate to SAH concurrently. The analysis showing an association of the intercept and the slope of SAH with mortality provided strong support for the hypothesis that individuals rely heavily on FL to assess their own health [10] and that FL may be in part responsible for the validity of SAH as a predictor of mortality and morbidity. The current evidence is consistent with multiple studies showing that FL are highly sensitive to changes in health status even when these changes are not conceptualized as disease [38]. Our data also suggest that trait affects, especially trait PA, modestly biased SAH ratings, which could account for errors in the association of SAH with health outcomes. This is not to argue that affective processes cannot impact SAH ratings or health-related outcomes; if they do so, however, it will likely be through more complex pathways than those tested in this study. For example, trait NA may have indirect effects on SAH ratings by influencing the perception and report of somatic changes [39]. Similarly, there is evidence that some aspects of trait PA, such as positive self-perceptions, are predictive of mortality [40]. The existence of significant residual variance in observed, and latent SAH scores indicates that there are other factors not examined in this study that might influence SAH ratings and the validity of these ratings as predictors of mortality. Given that people can process only a limited amount of information at a given time [41], we argue that there is a limited set of factors sensitive to health-related, negative and positive changes that provide valid and reliable input for making SAH judgments [13].
The findings have special significance for the CSM, which guided our analytic approach. A central postulate of the model is that an active process is involved in matching changes in somatic experience to underlying illness prototypes. People perceive themselves as sick or well depending upon the properties of symptoms and/or changes in function, such as the properties and location of symptoms (sharp, dull, throbbing; pain in chest, throat, or ear), and their duration and trajectory (i.e., fluctuating, getting worse), causal antecedents (e.g., exposure to spoiled food, cold/wet weather), and their response to efforts at control (e.g., headache did or did not clear with aspirin). Checks on the properties of symptoms and functional changes assess the match of individuals’ experiences to underlying prototypes. These prototype checks operate both automatically and deliberately, generating perceptions and judgments that a physical or functional change is due to an acute and benign condition from exposure to a particular pathogen or situational stress (e.g., headache that occurred during final exams), possibly chronic and serious with implications for the self (e.g., headache together with speech problems cause by a stroke), or inconsistent with any underlying CSM and in need of expert evaluation [22].
The present data identified functional limitations as a key prototype check for the self-system. Thus, a change that is unresponsive to various options for self-management and/or alterations in mood can result in a self-diagnosis of chronic change in physical and/or cognitive resources. This self-diagnosis, in turn, can affect self-assessments of health, use of health care, work and interpersonal relationships, and affective states. In short, SAH judgments are valid representations of health when based upon information reflective of factors integral to an individual’s physical health status such as functional changes or symptoms that are related to underlying pathology rather than emotionally induced and medical diagnoses [23].
The generalizability of these findings, and our interpretation of them, are limited by the following constraints. First, although the changes in SAH and FL were significant, from a clinical perspective, their magnitude over a 5-year period was modest. Thus, although the results are consistent with evidence showing that a majority of individuals experience slow declines in both psychological and physical health as they age [35], a different picture may emerge among individuals undergoing rapid declines in health. Second, caution is needed in generalizing these findings to the population at large, as our sample consisted of mainly well-educated, well-to-do, and healthy community-dwelling elderly adults. Replication in a population-based sample would provide strong support for our interpretation of the factors affecting self-appraisals. Third, a more detailed examination of the factors involved in updating SAH judgments will be relevant for understanding whether, and if so, under which conditions affective factors bias health-related judgments [14] and under what conditions they have direct and/or indirect effects on physical health (e.g., mortality) [42, 43].
Our findings have at least two potentially important implications for future research on the psychological processes underlying SAH judgments and for clinical practice. First, it would be worthwhile to develop laboratory studies similar to those used in expert systems research [44] to more precisely define how people use different types of information in making SAH judgments. Second, the absence of cross-lagged effects suggests a reciprocal association between SAH and both trait PA and FL. Future studies need to further examine the impact of SAH on these factors and the underlying mechanisms. Finally, it would be interesting and of clinical importance to determine if sets of individuals showing different SAH trajectories arrive at their ratings using the same or different processes than those proposed in the present analysis. Understanding these differences could be helpful in judging the clinical importance of these self reports.
References
Idler E, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997; 381: 21–37.
Lyyra T-M, Heikkinen E, Lyyra A-L, Jylhä M. Self-rated health and mortality: Could clinical and performance-based measures of health and functioning explain the association? Arch Gerontol Geriatr. 2006; 423: 277–288.
Dunning D, Heath C, Suls JM. Flawed self-assessment. Implications for health, education, and the workplace. Psychol Sci Public Interest. 2004; 53: 69–106.
Winter L, Lawton M, Langston CA, Ruckdeschel K, Sando R. Symptoms, affects, and self-rated health: Evidence for a subjective trajectory of health. J Aging Health. 2007; 193: 453–469.
Ferraro KF, Kelley-Moore JA. Self-rated health and mortality among Black and White adults: Examining the dynamic evaluation thesis. J Gerontol B Psychol Sci Soc Sci. 2001; 56B4: S195–S205.
Han B, Phillips C, Ferrucci L, et al. Change in self-rated health and mortality among community-dwelling disabled older women. Gerontologist. 2005; 452: 216–221.
Benyamini YEAL, Leventhal H. Elderly people's ratings of the importance of health-related factors to their self-assessments of health. Soc Sci Med. 2003; 568: 1661–1667.
Barger SD, Burke SM, Limbert MJ. Do induced moods really influence health perceptions? Health Psychol. 2007; 261: 85–95.
Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: Expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000; 55B2: P107–P116.
Barger SD. Do psychological characteristics explain socioeconomic stratification of self-rated health? J Health Psychol. 2006; 111: 21–35.
Singh-Manoux A, Martikainen P, Ferrie J, et al. What does self rated health measure? Results from the British Whitehall II and French Gazel cohort studies. J Epidemiol Community Health. 2006; 604: 364–372.
Benyamini Y, Leventhal EA, Leventhal H. Self-assessments of health: What do people know that predicts their mortality? Res Aging. 1999; 213: 477–500.
Williams PG, Wasserman MS, Lotto AJ. Individual differences in self-assessed health: An information-processing investigation of health and illness cognition. Health Psychol. 2003; 221: 3–11.
Loewenstein G. Hot–cold empathy gaps and medical decision making. Health Psychol. 2005; 244 Suppl: S17–S22.
Abelson RP. Computer simulation of ‘Hot’ cognition. In: Tomkins SS, Messic S, eds. Computer Simulation of Personality: Frontier of Psychological Theory. New York: Wiley; 1963: 277–298.
Schwarz N, Strack F. Reports of subjective well-being: Judgmental processes and their methodological implications. In: Kahneman D, Diener E, Schwarz N, eds. Well-Being: The Foundations of Hedonic Psychology. New York: Sage; 1999: 61–84.
Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. J Pers Soc Psychol. 1991; 611: 132–140.
Bower GH. Mood congruity of social judgments. In: Forgas JP, ed. Emotion and Social Judgments. Oxford: Pergamon; 1991: 31–53.
Chipperfield JG, Perry RP, Weiner B. Discrete emotions in later life. J Gerontol B Psychol Sci Soc Sci. 2003; 581: P23–P34.
Leventhal H, Diefenbach M, Leventhal EA. Illness cognition: Using common sense to understand treatment adherence and affect cognition interactions. Special Issue: Cognitive perspectives in health psychology. Cogn Ther Res. 1992; 162: 143–163.
Henderson CJ, Hagger MS, Orbell S. Does priming a specific illness schema result in an attentional information-processing bias for specific illnesses? Health Psychol. 2007; 262: 165–173.
Leventhal H, Forster R, Leventhal E. Self-regulation of health threats, affect, and the self: Lessons from older adults. In: Aldwin CM, Park CL, Spiro A III, eds. Handbook of Health Psychology and Aging. New York: Guilford; 2007: 341–366.
Idler E, Leventhal H, McLaughlin J, Leventhal EA. In sickness but not in health: Self-ratings, identity, and mortality. J Health Soc Behav. 2004; 453: 336–356.
Wolinsky FD, Armbrecht ES, Wyrwich KW. Rethinking functional limitation pathways. Gerontologist. 2000; 402: 137–146.
Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: The association revisited. Diabetes Care. 2005; 2810: 2378–2383.
Johnson RJ, Wolinsky FD. The structure of health status among older adults: Disease, disability, functional limitation, and perceived health. J Health Soc Behav. 1993; 342: 105–121.
Shanks JM. The current status of computer-assisted telephone interviewing: Recent progress and future prospects. Sociol Method Res. 1983; 122: 119–142.
Bollen KA, Curran PJ. Autoregressive Latent Trajectory (ALT) Models A Synthesis of Two Traditions. Sociol Method Res. 2004; 323: 336–383.
Asparouhov T, Masyn K, Muthen B. Continuous time survival in latent variable models. Paper presented at Joint Statistical Meeting ASA section on Biometrics, 2006; Seattle, WA.
Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002; 72: 147–177.
Yuan K-H, Bentler PM. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociol Method. 2000; 30: 165–200.
Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. Hoboken: Wiley; 2002.
Satorra A, Bentler P. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001; 664: 507–514.
Burnham KP, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Method Res. 2004; 332: 261–304.
Aldwin CM, Spiro A III, Levenson MR, Cupertino AP. Longitudinal findings from the normative aging study: III. Personality, individual health trajectories, and mortality. Psychol Aging. 2001; 163: 450–465.
Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. J Pers Soc Psychol. 2001; 801: 136–151.
Hastie R, Park B. The relationship between memory and judgment depends on whether the judgment task is memory-based or on-line. Psychol Rev. 1986; 933: 258–268.
Greiner PA, Snowdon DA, Greiner LH. Self-rated function, self-rated health, and postmortem evidence of brain infarcts: Findings from the Nun Study. J Gerontol B Psychol Sci Soc Sci. 1999; 544: S219–S222.
Mora PA, Halm EA, Leventhal H, Ceric F. Elucidating the relationship between negative affectivity and symptoms: The role of illness-specific affective responses. Ann Behav Med. 2007; 341: 77–86.
Levy BR, Slade MD, Kunkel SR, Kasl SV. Longevity increased by positive self-perceptions of aging. J Pers Soc Psychol. 2002; 832: 261–270.
Halford GS, Baker R, McCredden JE, Bain JD. How many variables can humans process? Psychol Sci. 2005; 161: 70–76.
Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005; 1316: 925–971.
Barefoot JC, Brummett BH, Helms MJ, et al. Depressive symptoms and survival of patients with coronary artery disease. Psychosom Med. 2000; 626: 790–795.
Gobet F. Expert memory: A comparison of four theories. Cognition. 1998; 662: 115–152.
Acknowledgments
This study was funded by Grant NIA AG03501 from the National Institute on Aging. The authors were assisted by grant NIA AG023958 during the writing of this article. We would like to thank Melissa Crouch, Susan Brownlee, and Frances Sisack for their assistance on various aspects of the research reported in this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mora, P.A., DiBonaventura, M.D., Idler, E. et al. Psychological Factors Influencing Self-Assessments of Health: Toward an Understanding of the Mechanisms Underlying How People Rate Their Own Health. ann. behav. med. 36, 292–303 (2008). https://doi.org/10.1007/s12160-008-9065-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-008-9065-4