Abstract
Here, a cost-effective route for the preparation of biodiesel (BD) from waste cooking oil (WCO) as an alternative eco-friendly fuel and lubricant via catalytic transesterification process using simple base catalyst was demonstrated. Physical and chemical characterization based on FT-IR, 1H-NMR, and 13C-NMR spectroscopy reveals that free carboxylic acid functionality successfully alters to methyl ester of same hydrocarbon chain during the reaction. The BD was characterized by its physical, fuel characteristics including density, viscosity, acid value, flash point, pour point, and foaming tendency, and compared with existing diesel fuel in accordance with ASTM test standard. Prepared BD showed excellent fuel efficiency with higher flash (142 °C) and fire point (147 °C) than the existing diesel fuel. In order to use these synthetic biodiesel esters as advanced green lubricants, their lubrication behavior was tested and compared with hydrocarbon base oil hexadecane (HD). The macrotribological results showed the biodiesel significantly reduced the coefficient of friction by a maximum ~ 43% and specific wear rate ~ 71% in comparison with that of HD at variable applied load. The lubricating efficiency of prepared BD was found to be a similar trend in microtribometric experiments with reciprocating sliding motion under variable loads with similar contact pressure (Pm ~ 1.36–1.95 GPa) as demonstrated in macrotribological rotating motion. Due to polar nature of biodiesel, it deposits on the contact interface and provides a thick molecular film, which may be responsible for enhanced tribological behavior compared with pristine hydrocarbon oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The petroleum-based energy resources are decreasing day by day due to the lack of cyclization methods. Therefore, conservation of non-renewable energy resources has become now an attractive issue [1, 2]. However, use of petroleum-based energy sources creates major environmental pollution problems and emission of carbon dioxide, greenhouse gases, and various organic compounds, which are responsible for climate change [3,4,5]. Therefore, the use of green fuels instead of petroleum fuels is an utmost challenge for environmental concern in the twenty-first century [5]. In present days, necessity of fuels is increasing enormously due to the increase in industrialization and transportation issues. Therefore, renewable biofuel will be the best alternatives for energy, which can be obtained from plants and domestic, agricultural, and industrial wastes [6,7,8]. Biodiesel is one of the most important biofuel, which is a safe and replaceable alternative fuel of traditional petroleum fuel. It can be used as a clean-burning fuel, unmodified diesel engine, and fuel components for existing diesel engine [1, 5]. Biodiesel is known as a completely renewable fuel source and can be easily made from plant-based materials like vegetable oil (edible, non-edible, or waste oils), animal fats (mostly edible fats or waste fats), or WCO [9].
In recent years, the waste cooking oil (WCO) has been used as a feedstock for the preparation of biodiesel (BD) for optimum waste management and energy conservation. The repeated use or frying of cooking oil increases the free fatty acids and is no longer suitable for consumption [10], thereby a concern for its disposal. The irrational disposal of WCO creates water and soil pollution, which is responsible for adverse human health, and environmental pollution concern [9, 10]. It is estimated that the amount of produced WCO is about 600,000 t in India [11]. If this large amount of WCO can be recycled properly rather than disposal, it will be benefited both energetically and environmentally [12,13,14,15]. Presently, several research works were reported for the preparation of biodiesel and its characterization from various vegetable oil (majorly such as soybean, castor, palm, rapeseed, corncob, olive oil), non-edible seeds, and WCO [2, 11, 16]. Among all the vegetable oil, palm oil has more efficiency to produce biofuel feedstock due to similar chain length as fossil oil. However, the use of vegetable oil as a fuel creates much problem due to high viscosity, which is responsible for the higher smoke emissions, improper combustion, and air-fuel mixture formation and atomization [17]. The long-term use of vegetable oil as fuel creates additional problems such as nozzle coking, carbon atom deposition in parts of the machine [18, 19]. The use of biodiesel as a fuel in the vehicle without any engine modification reduces emission of environmentally harmful gases significantly and keeps the environment pollution-free [1, 16, 20, 21].
There are so many reports for the preparation of biodiesel such as microemulsions in diesel fuel, blending of diesel fuel, and thermal cracking of vegetable oil/animal fats and transesterification of animal or vegetable oil [22,23,24,25,26]. Among all the methodologies, transesterification is one of the crucial method, where ester is formed using alcohol and suitable acidic/basic catalysts through alcoholysis method. Ferrero et al. [25] reported that CaO could be effectively used as a catalyst for the production of biodiesel through transesterification reaction using methanol as alcohol. Mahesh et al. [26] further reported the solid-based catalytic transesterification reaction with potassium bromide and calcium oxide as heterogeneous catalysis. Heterogeneous catalysis is preferred in transesterification process due to its simple separation process and reduced soap formation during reaction [27]. However, the key challenge for commercialization of biodiesel from animal fats or vegetable oil is the cost-effectiveness. The cost of raw materials plays a vital role in biodiesel production. An earlier study reported that around 70–95% production cost directly depended on used raw materials for biodiesel production [28]. However, the production cost can be minimized by using WCO as a raw material. Girotto et al. [29] investigated the waste cyclization of the kitchen and suggested the improvement of waste processing of foods for cyclization and management of these wastes for energy recovery as well as biofuel production. Li et al. [30] reported the importance and feasibility of biodiesel production using waste cooking oil using a heterogeneous catalyst and evaluated a low-cost production pathway of biodiesel. Tsai et al. [31] also reported an analytical procedure for the preparation of biodiesel from waste cooking oil for mainly economical incentives and environmental protection. Karthickeyan et al. [12] demonstrated a simple route for biodiesel preparation from the waste pomegranate fruit seed oil through transesterification reaction pathway to investigate the effect of prepared biodiesel with engine operating parameter in diesel engine. Nanthagopal et al. [6] also synthesized Calophyllum inophyllum methyl ester (biodiesel) through a simple two-step transesterification reaction pathway and observed the engine performances and gas emission characteristics in diesel engine with the prepared biodiesel. Above studies showed that increase in blend ratio of synthesized biodiesel was found to improve significantly combustion, performance characteristics, and reduced gas emission (oxides of nitrogen, smoke, and carbon dioxide).
There are only few studies cited in the open literature related to tribological behavior of mechanical components with biodiesel. Recently, a fuel injection equipment (FIE) test rig was designed, and the wear characteristics of the components used by a single cylinder diesel engine were investigated using biodiesel. The wear of FIE components was found to be relatively lower with biodiesel compared with the commercial diesel fuel usage in the test rig [32]. The long-term tribomechanical stability of mechanical components using biodiesels has not been explored in details. The test fuels with poor lubricity can increase the friction and wear of the engine components and can even lead to disastrous engine failure. Although many works has been reported for the preparation/physico-chemical characterization/fuel property evaluation of biodiesel, limited focus has been given to the lubricating efficiency with respect to the tribochemical phenomena of biodiesel as a green fuel and lubricant. The objective of this study is to develop biodiesel through a facile synthetic route from waste cooking oil and evaluate their tribological behavior compared with that of the hydrocarbon oil with similar carbon chain length as biodiesel. The lubricating efficiency of the biodiesel was compared with lubricating hydrocarbon base oil hexadecane. Thereafter, wear and deposits formed on the contact interface were experimentally evaluated, and compared with baseline hexadecane oil. Optical microscopy and FE-SEM images were taken to evaluate the changes in the surface texture of the tribo components and the wear scar of the counterface ball while using different test fuels under variable tribological conditions.
Materials and Methods
Materials
Used cooking oil (palm oil) collected from the canteen (CSIR-CMERI canteen) for the starting material of biodiesel. Sodium hydroxide (AR grade, Merck), methanol (AR grade, Merck), absolute sodium chloride (AR grade, Merck), anhydrous sodium sulphate (AR grade, Merck), deionized water (DI, AR grade, Merck), and ethyl acetate (AR grade, Merck) were purchased and used as chemicals for preparation of biodiesel. Ethanol (AR grade, Merck) and acetone (AR grade, Merck) were used for cleaning purpose. For tribological experiments, commercially available hardened 440 C stainless steel plate (contains 0.15% carbon, 1% manganese, 0.04% phosphorus, 0.03% sulfur, 1% silicon, 11.5–13.5% chromium and remaining iron (hardness (HV) = 510, elastic modules = 193 GPa, Poisson’s ratio = 0.27, RMS roughness ~ 5 nm) were used as the disc materials. Chromo steel balls of diameter 10 mm and 6 mm (RMS roughness ~ 5 nm) were purchased from commercially available source for ball-on-disc tests. Hexadecane (99%, Sigma-Aldrich) was purchased and used as a comparable base lubricating oil to prepared biodiesel for tribological investigation.
Chemical Synthesis of Biodiesel from Waste Cooking Oil
In this study, chemical synthesis of BD from WCO has been performed through base catalytic transesterification process [2, 11, 26]. Before transesterification process, pre-treatment of WCO was carried out to reduce the high acid value and removal of food as well as undesired particle contamination from WCO. For pre-treatment of WCO, about 1.5 l of used cooking oil was taken in a beaker, and 5 g of activated carbon was added to the WCO to adsorb the excess FFA content [26]. It was then mixed properly with a magnetic stirrer at 200 rpm for 60 min at ambient condition. Then the oil was filtered to separate out the impurities and a suspended particle contents in the oil and used for biodiesel preparation. For transesterification process, about 800 ml of clean (pre-treated oil) WCO was taken in a 2000-ml beaker and heated at 110 °C for 1–2 h in a preheated oil bath to remove the water molecules. Simultaneously, 3.5 g of sodium hydroxide palette was taken in another 500-ml beaker. Then 60 ml of methanol was added to the NaOH-containing beaker. After that, the mixture was placed on a magnetic stirrer and stirred with a magnetic stir bar at 500 rpm for 20 min until the total NaOH dissolves. Then the methanolic NaOH solution was added to the oil-containing beaker. Then the resulting mixture was mixed vigorously using a magnetic stirrer at 1000 rpm for 20 min at 60 °C in a preheated oil bath. The vigorously stirring procedure is necessary to avoid layer separation. Then the reaction mixture was transferred to a three-neck flat round-bottom flask attached to a condenser. The reaction mixture was refluxed for 3 h at 80 °C with continuous mechanical stirring using a magnetic stirrer at 500 rpm. Then, the final reaction mixture was cooled at normal temperature followed by addition of 30 ml ethyl acetate and 40 ml water to adjust the solution pH ~ 8–9. Then the mixture was transferred to a separating funnel for phase separation. The separating funnel was left for 2 h for complete organic phase separation. The upper phase color was a pale yellow color which contained biodiesel, whereas the lower layers were reddish-brown in color which mostly contained glycerine and unreacted counterparts (excess methanol, unreacted sodium hydroxide, water, and salts). The lower parts of the separating funnel were drained, and the biodiesel was collected. Then the resulting biodiesel was washed with 30 ml of DI water to remove remaining byproducts and unreacted contaminants (unreacted reagents, glycerine, soap). For accurate washing, the biodiesel was swirled for 2 min to completely dissolve the unwanted parts. Then aqueous layer was again separated using a separating funnel. Then the resulting upper layers were collected as biodiesel. To get fresh biodiesel, the product was allowed to be vacuum filtered with a Buchner filtration apparatus for two times. Then about 1 g of anhydrous sodium sulfate was added and swirled gently for 2 min to remove the remaining trace amount of water contaminants. The separation and vacuum filtration were continued for two times to get fresh biodiesel and collected in a clean and dry container for physico-chemical, fuel efficiency, and tribological characterization. Step-wise synthetic procedure has been presented schematically in Fig. 1.
Structural and Chemical Characterization Techniques
The FT-IR spectra of the filtered used cooking oil and produced biodiesel were acquired by a Perkin-Elmer Spectrum 100 spectrometer. All FT-IR spectra reported here were referenced to the liquid cells of KBr pellet and acquired over 100 scans at 4 cm−1 resolution. The spectral analyses were carried out with the Spectrum v10.00 software (Perkin-Elmer). 1HNMR spectral analyses of WCO and BD were performed on a Bruker AVANCE at 500 MHz instruments at variable temperature. The 13C NMR and corresponding DEPT-135 spectral analysis were investigated with Bruker AVANCE at 126 MHz. All chemical shifts are reported here in ppm relative to the residual solvent peak. NMR spectra were acquired in CDCl3 and passed through a plug of basic alumina; individual peaks have been reported as: multiplicity (where s = singlet, d = doublet, t = triplet, q = quartet, and m = multiplet), integration and coupling constant in Hz.
Fuel Property Characterization Techniques
The fuel properties of prepared biodiesel have been evaluated as per various ASTM standard investigation methods under laboratory conditions, and the fuel efficiency of prepared biodiesel was compared with that of existing diesel fuel [2, 33].
Viscosity Measurement
Viscosity and viscosity index of the WCO and BD were carried out using SPECTRO INC., Automatic Viscometer. Viscosities of the samples were measured by an automatic viscometer according to ASTM D445 standard at 40 °C [33]. During the experiments, the drying and cleaning processes were continued automatically with n-heptane solvent followed by the introduction of measuring samples (0.3 to 0.6 ml) into the vertical pipe for the investigation.
TAN (mg KOH/g)
Acid number and base number present in the WCO and BD were investigated by TAN analysis using Metrohm-877 Titrino Plus at 20 °C. The results were collected from titrimetric analysis with an indicator using manual dispensing control and 6.3026.220 exchange unit (20 ml). The value of TAN was calculated automatically from the dose volume. The tests were carried out by ASTM D664-11 and BS DIN EN-12634 Standards.
Flash/Fire Point
The measurements of flash point and fire point of WCO and BD were carried out using Pensky-Martens Closed Cup Apparatus (manual). In this measurement, a brass test cup with standard specified dimensions was filled up to inside marking with the tested samples and covered followed by heating with an ignition source. The experiments were carried out by ASTM D93 standard with the apparatus [2].
Foaming Tendency/Stability
The foaming tendency of the WCO and BD was acquired by the foaming test apparatus with ASTM D892-IP 146 standard.
Demulsibility
Demulsibility (the ability of oil and water to separate from each other) of WCO and prepared BD was measured by an emulsifying apparatus with ASTM D1401 standard. About 40 ml of WCO/BD samples were mixed and stirred with 40 ml DI water for 10 min at 90 °C followed by settling for 1 h.
Copper Corrosion
Copper corrosion tests were carried out by the copper corrosion apparatus according to ASTM D130 standard. In these experiments, the emery-polished copper strip was immersed in the tested sample according to a standard method parameter.
Specific Gravity
The specific gravity of the samples was measured by a density hydrometer as per the ASTM D891-09 standard at 25 °C.
Tribological Characterization
Tribological experiments were performed by a macrotribometer (DUCOM, Bangalore, India) and a microtribometer (UMT-2, Bruker, USA) with ball-on-disc contact geometry. For macrotribological ball-on-disc tests, the disc samples (RMS roughness ~ 400 nm) were polished through variable grit-size (400, 800, 1200, and 2000) emery paper. Then the disc samples were cloth polished with diamond paste of grade 1–3 μm using a double-disc polishing machine (BAINPOL, Chennai Metco Pvt. Ltd., India) followed by cleaning with ethanol/acetone for several times prior to the tribological experiments. A 10-mm diameter steel ball was used as a counterface surface. All the macrotribological tests were carried out at 0.4 m/s rotating speed for 1 h. Herein, all the test methods were adopted with ASTM G 99 standard. The load sensor ((LVDT, range = ± 500 μm, resolution 1 μm), which is attached to the ball holder (resolution 0.1 N), provided the frictional force. The data were collected by a software as coefficient of friction (COF) with sliding time using the formula, μ = F/N, where μ is the coefficient of friction, and F and N are ascribed as frictional force and applied load respectively.
Microtribological experiments of prepared BD and hexadecane (HD) were performed by a Universal Mechanical Tester (UMT-2, BRUKER, USA) with reciprocating sliding motion. All experiments were performed using a 2-axis friction/load sensor (DFH-50, range ~ 5–500 N, resolution ~ 25 mN) for 1 h. A 6-mm diameter chrome steel ball (RMS roughness ~ 5 nm) was attached to the ball holder and used as a counterface sliding surface with a reciprocating sliding speed of 5 mm/s using a variable loading condition. The frictional force and wear resistivity were obtained from the load sensor as COF value and wear depth using z-axis displacement respectively. The tribological test conditions are presented in Table 1. All sets of tribological experiments were performed five times, and average data have been reported with relative error for both COF and wear profile. The wear phenomena of the macrotribological ball-on-disc tests have been utilized to evaluate the specific wear rate of counterface ball surfaces. The specific wear rate has been calculated from the corresponding wear scar diameter (WSD) of used balls. The specific wear rate generally expressed as (wear volume/(load*total sliding distance), mm3/N-m). To calculate the specific wear rate, total wear volume has been determined from WSD of counterface ball through the following equations [34]. \( V=\left(\frac{\pi h}{6}\right)\left(\frac{3{d}^2}{4}+{h}^2\right) \)and\( h=r-\sqrt{\left({r}^2-\frac{d^2}{4}\right)} \), where V represents the wear volume, r is the radius of steel ball, and d is the corresponding WSD. Post-tribological morphology of the tribo film formed during sliding on used ball and disc was further explored to establish friction mechanism. The optical images of ball and wear scar diameter (WSD) were investigated by an Nikon optical microscope (Micropublisher 3.3 RTV CCD camera, Canada). The morphological status of the sliding disc surfaces was obtained by field emission scanning electron microscopy (FE-SEM, sigma HD, Carl Zeiss, Germany).
Results and Discussion
Chemical Characterization of Prepared Biodiesel
FT-IR Spectral Analysis
Characteristics FT-IR spectral analysis of WCO and synthesized BD have been shown in Fig. 2. The FT-IR spectra of WCO and BD were found to be identical with similar chemical functionality. The carbonyl (−C=O) functionality was observed between the region 1800 cm−1 and 1700 cm−1 for both the samples. The C=O stretching frequency of used palm oil was observed at 1750 cm−1 due to the presence of mainly FFA present in the sample. However, after transesterification of WCO, the C=O stretching frequency of methyl ester back shifted at 1742 cm−1 [35]. The fingerprint region (1500–900 cm−1) of the spectrum showed many chemical differences between used palm oil and synthesized biodiesel. The characteristics peak ~ 1460 cm−1 was assigned for the bending vibration of –CH2 and –CH3 groups for both the samples. However, another characteristic peak at 1440 cm−1 was observed for –CH3 asymmetric bending in case of biodiesel; however, that peak was absent in WCO [36]. The peak at 1197 cm−1 was attributed to the stretching vibration of –OCH3 of BD (attributed from the methyl ester), which was absent in the used oil [36]. The characteristic –C−O stretching bands were represented at 1169 and 1245 cm−1 for both the samples. However, the peak intensity of biodiesel reduced significantly due to the removal of triglyceride and FFA and formed methyl ester functionality in biodiesel [37]. The peak position at 3009 cm−1, assigned for = C−H and 1650 cm−1 mainly for the C−C double bond, was observed in both the samples. But the peak intensity for BD was reduced which described the reduction of the double-bond character during chemical reaction in case of biodiesel. The saturated C–C bond (−C−H stretching frequency) was observed at the peak position 2928 cm−1, 2854 cm−1, and 721 cm−1 with lower intensity for BD than WCO. The lowering of peak intensity again confirmed the reduction of chain length during transesterification process.
1H NMR Spectral Analysis
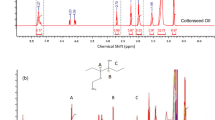
The used palm oil and prepared biodiesel were investigated with 1H NMR spectra (the 1H NMR spectra was given in Supplementary materials, Fig. S1). The terminal methyl (−CH3) and methylene protons (−CH2) were observed at the peak position 0.87 and 1.25 ppm as a multiplet for both the samples. For the WCO sample, the third carbon-attached proton of −CO−CH2−CH2− functionality was originated at 1.62 ppm. Peak positions at 2.01 ppm and 2.32 ppm also appeared for the allylic proton (−CH2−CH=) and the proton next to the carbonyl linkage (−CO−CH2−) in the fatty acid chain in WCO (Fig. S1a). The peak position at 4.15–4.32 ppm was attributed to the proton of glyceride –CH2 functionality [38]. The multiplet proton signal corresponding at 5.30–5.50 ppm was observed due to the presence of olefinic protons (−CH=CH−) in the WCO (Fig. S1a).
The characteristic 1H NMR spectra of prepared BD from used palm oil are shown in Fig. S1b. The similar spectral features of biodiesel were obtained at the spectral region 0.5–3.0 ppm. This characterization is mainly due to the presence of unsaturated long alkyl chain moieties in BD. The major discrepancy was observed for the peak position of 4.15–4.32 ppm, which was due to the glycerol moieties of WCO (–OCH2 protons). These peaks were vanished in the BD after chemical treatment. Another important observation was the signal for the methyl ester. A singlet signal at the region of 3.69 ppm was observed for methoxy proton (–OCH3) of BD sample which was not observed for WCO (Fig. S1b) [39]. The olefinic proton signals were retained after BD formation from used unsaturated fatty acid in palm oil. Therefore, the 1H NMR spectral study corroborated regarding the evidence of BD formation from WCO through methyl ester formation.
13C NMR Spectral Analysis
13C NMR spectral analyses of the WCO and prepared BD are shown in Fig. S2 and Fig. S3 respectively. 13C NMR has some advantages over 1H NMR spectral study due to the large ranges of chemical shifting. As a result, the signals are sharp and well-resolved. Figure S2 shows the13C NMR spectra with corresponding 13C DEPT-135 NMR spectrum of WCO. The sharp signal of chemical shifts position of WCO showed clearly the presence of triglyceride and its corresponding fatty acid chain. The chemical shift of WCO for the presence of saturated and unsaturated fatty ester at 173.27 ppm (−COOCH2) and 172.86 ppm (−COOCH) was displayed for fatty esters of saturated and unsaturated triglyceride moieties (Fig. S2a). The olefinic carbon (−CH=CH–) signals were also appeared at 127.9–130.24 ppm (Fig. S2a). The carbon signals of –OCH2 and –OCH for triglyceride were appeared at 68.89 ppm and 62.11 ppm respectively [40]. The long alkyl fatty acid methyl and methylene carbon appeared for WCO at 14.1–31.53 ppm.
13C NMR spectral analysis was continued further with the DEPT-135 spectral analysis (Fig. S2b). In addition, normal 13C NMR spectra are broadband decoupled, whereas DEPT (distortion less enhancement of polarization transfer) can be used to give similar information to an off-resonance decoupled spectra, i.e., the number of attached hydrogen. In DEPT-135 signals, the odd number–attached hydrogen-containing carbons have positive phase (up) and those with an even number of H have a negative phase (down). Figure S2b showed the DEPT-135 spectral analysis of WCO. The terminal methyl carbon was observed only the signals ~ 13.99–14.20 ppm for triglyceride and free acid chains. The –CH2 carbon signals of the same chains appeared at below 34.00 ppm. The characteristics CH2 and CH carbon of glyceryl functionality at 68.89 and 62.11 ppm signals were found in an up and down configuration. The peaks at 173.27 and 172.86 were absolutely disappeared due to ester carbonyl peaks.
13C NMR spectrum of biodiesel is shown in Fig. S3a. The identification of BD was characterized by the ester signal at 174.33 ppm. The olefinic carbon signals at 127–130 ppm were retained as precursor oil (Fig. S3a). However, the important characterization for the preparation of biodiesel was identified as the methoxy carbon (−OCH3) of ester at 51.44 ppm [41]. But the carbon signal of –OCH2 and –OCH for BD were totally disappeared during formation of methyl ester (Fig. S3a). The methyl and methylene carbon signal appeared in the same position, which described the formation of methyl ester from its respective fatty acid chains [41]. Corresponding DEPT-135 spectral analysis of BD from WCO was carried out and depicted in Fig. S3b. This spectral analysis also confirmed the preparation of methyl ester during transesterification reaction. DEPT spectrum provided an authentic observation about the nature of carbon multiplicities and types of carbon (CH3, CH2, CH, and quaternary carbon) in the product. The terminal methyl carbon was observed with the signals ~ 13.99–14.20 ppm, and –CH2 carbon signals corresponding to below 34.00 ppm was also observed like the WCO signal for the same fatty acid carbon chains (Figs. S2a, S3a). The characteristic CH2 and CH carbon of glyceryl functionality at 68.89 and 62.11 ppm signals totally vanished, whereas signal at 51.44 ppm appeared for methyl ester (−OCH3) carbon (Fig. S3a). The olefinic carbon (−CH=CH−) signals retained at their respective position ~ 127.9–130.24 ppm. The corresponding ester signal at 174.33 ppm disappeared due to the quaternary nature. Therefore, the 13C NMR spectrum confirmed the formation of biodiesel from WCO through transesterification reaction pathway.
Investigations of Fuel Efficiency of Prepared Biodiesel
In this work, non-edible WCO (palm) oil was converted into BD using transesterification reaction pathway. The fuel efficiency of the prepared BD was investigated and compared with existing diesel fuel as depicted in Table 2. The characteristic experimental results have been investigated based on ASTM standard test procedure under laboratory condition. Viscosity of prepared BD at 40 °C was observed ~ 3.1 cSt, slightly higher than existing diesel fuel (~ 2.57 cSt) and within the ASTM limits [5, 42]. TAN analyses were performed for acid number present in the WCO and BD. Relatively high acid value of 12.86 mg KOH/g was observed for WCO sample due to the appearance of FFA contents during frying process. Therefore, the WCO was pre-treated through continuous stirring with activated carbon to remove the excess FFA content. Recently, Aboelazayem et al. prepared a biodiesel from WCO having a relatively higher acid value of 18 mg KOH/g through non-catalytic transesterification using supercritical methanol [33]. However, after transformation to BD, the acid value reduced significantly (Table 2), indicating removal of FFA to produce ester. Pensky-Martens Closed Cup apparatus was used to observe flash point and fire point of WCO and BD. Prepared biodiesel showed higher flash and fire point than the existing diesel fuel (Table 1), indicating excellent fuel efficiency of prepared BD [5, 43]. The foaming tendency of the WCO and BD was investigated to identify the performance of fluid life and as a lubricant. The foaming tendency of a fluid minimizes the lubricating efficiency in high-speed condition and system performances. The resulting volume indicates the volume of foam after flowing air at a constant flow rate in 190 ml of oil sample at standard temperature, shown in Table 1. Prepared BD exhibited comparably less foaming tendency, indicating better fuel as well as lubricating efficiency. The demulsibility results are displayed in Table 1 as A-B-C(D) format, where A/B is the volume of oil/water and C/D is the volume of emulsion/settling time in minutes. Prepared BD exhibited comparably less demulsibility tendency than the existing diesel fuel (Table 1). Copper corrosion result of BD and existing diesel fuel also have been found to be similar, indicating excellent fuel efficiency of prepared BD. From the comparative fuel efficiency study, prepared biodiesel showed better fuel efficiency and higher flash and fire point than the existing diesel fuel.
Tribological Investigation
Macrotribological experiments were carried out by adding prepared BD on the disc substrate as a lubricant. In ball loading experiments, the ball holder, which contains 10 mm steel ball, is pressed against the rotating steel disc to generate mean Hertzian pressure ~ 1.4 GPa at 30 N normal load. The tests were performed under three different loads (30 N, 50 N, and 80 N) for the prepared BD for 1 h. The main focus here was to demonstrate how the BD will be able to control the coefficient of friction (COF) and wear of the counter surface with respect to the same chain length–lubricating base oil, hexadecane (HD). The variation of COF with time for the BD is displayed in Fig. 3a with different loading conditions (30 N, 50 N, and 80 N). At 30 N (Pm ~ 1.4 GPa) loading condition, biodiesel showed a COF of ~ 0.07 at the starting, but with sliding time, this value gradually decreases to ~ 0.05. Whereas with increasing normal load, 50 N (Pm ~ 1.65 GPa) and 80 N (Pm ~ 1.93 GPa), the COF value slightly increases and starts from 0.075 to gradually decreased ~ 0.065.
To explore the lubricating efficiency of biodiesel, the tribological performances of HD were also investigated in same loading condition, since the prepared BD (methyl ester of free palmitic acid) has same carbon chain length as HD. Figure 3b showed the COF value with respect to sliding time from the ball loading tribometric study for HD at variable loading conditions (30 N, 50 N, and 80 N). The results showed a considerable higher COF for HD compared with that for BD. The initial COF displayed ~ 0.08 (Fig. 3b), but with increasing sliding time, COF value gradually increases to ~ 0.12–0.14. It can be seen from Fig. 3 that the COF for BD was decreased by ~ 43% compared with that of HD in all loading conditions. The frictional trend of BD and HD was found to show divergent behavior to each other with reference to sliding time (Fig. 3a, b). With increasing sliding time, the COF decreased gradually for BD, which explained the accumulation of the long carbon chain esters (palmitic ester) in BD at the contact during sliding. The polar esters could effectively reduce friction by depositing in the contact interface to form a continuous stable antifriction film, which was reported earlier [44]. However, hydrocarbon chain containing HD was unable to adhere on the rubbing metal surface and started to get removed from the interface with increasing sliding time, which increased the COF values.
The observed frictional behavior was strongly corroborated with the ball wear scar diameter (WSD) and the corresponding specific wear rate value. The optical images of counterface ball and WSD at 30 N load for BD and HD have been shown in Fig. S4. It is found that the WSD of BD showed lower value ~ 625 μm compared with that of HD (~ 715 μm) (Fig. S4a and S4b), which supported the frictional data (Fig. 3). With increasing pressure, although the WSD value for BD increases, the WSD value was found to be lower compared with that of HD in all loading conditions (Fig. 4a). Furthermore, the wear phenomena was correlated with the frictional response. The wear scar diameter of the macrotribological ball-on-disc tests has been utilized to evaluate the specific wear rate of counterface ball surfaces. To calculate the specific wear rate, total wear volume has been determined from WSD of counterface ball through the following equations as given earlier [34]. Figure 4b depicted the wear rates of BD and HD in variable loading conditions. Ester containing BD was found to have a lower wear rate (~ 3.47 × 10−8 mm3/N-m for 30 N, ~ 2.83 × 10−8 mm3/N-m for 50 N, and ~ 2.10 × 10−8 mm3/N-m for 80 N load) compared with HD in all loading conditions (Fig. 4b). It can be seen from Fig. 4b that at 30 N load, the specific wear rate of the worn steel ball for BD was decreased by ~ 71% compared with that of the worn steel ball for HD. For the BD, the lower wear rates at higher loads show the excellent lubricity of the synthesized biodiesel, and this might be due to the polar-imparting O atoms which formed surface-active antifrictional films and accumulation of the palmitic ester into the interfaces as described earlier.
Furthermore, the tribological efficiencies of prepared BD and HD were investigated using ball loading microtribometer with reciprocating sliding motion. The experiments were performed under variable loads, 10 N, 20 N, and 30 N, with a similar contact pressure (Pm ~ 1.36–1.95 GPa) to that used in the macrotribometric experiments using 5 mm/s reciprocating sliding speed (Fig. 5). Under reciprocating sliding condition, the frictional behaviors of BD and HD were found to be a similar trend as demonstrated in macrotribological rotating motion (Fig. 3). Prepared BD showed lower COF (~ 0.1–0.11) than that of HD (~ 0.12–0.14) under all loading condition (Fig. 5a, b). As described earlier, BD containing palmitic ester, which forms surface-active adhesive film and accumulated in the sliding interfaces and reduced the COF compared with that of HD (Fig. 5c). With increasing pressure, the surface-active film might get deteriorated gradually to provide relatively higher COF compared with that of low contact pressure. However, in case of HD, the liquid film that formed at the initial sliding starts to deplete as the pressure builds up. This might be due to the poor adhesion of the hydrophobic hydrocarbon chain to the steel surfaces and is unable to form surface-active transfer film; as a result, the COF increases with increasing normal load (Fig. 5b).
The frictional profile has been further correlated with the corresponding wear depth data with sliding time for BD and HD in all loading conditions (Fig. 6). The wear depth profile was found to be in an order with corresponding COF data (Figs. 5 and 6). Herein, HD showed a wear depth up to ~ 1.5 μm at 30 N load, whereas with increasing normal load, the wear depth value increased to ~ 6 μm (Fig. 6b), which concluded that the deposited liquid film may not be effective and may get removed from the interface at higher sliding time and contact pressure. However, a negative wear depth has been observed for BD in all loading conditions (Fig. 6a). The negative wear depth explained the validation of accumulation for the palmitic ester chains and formation of surface-active adhesive film, which effectively reduces the COF (Fig. 5a).
The post tribological FE-SEM images of the worn tribometer tracks generated with 10 N (Pm ~ 1.36 GPa) load for both the samples were further investigated to establish the above tribological results (Fig. 7). As shown in Fig. 7a, the worn track of the BD displayed a continuous oilier film than HD-generated worn track (Fig. 7b) with significantly lower track width. The corresponding high-magnification FE-SEM images of the tribo film for BD (Fig. 7c) showed a stickier, thick adhesive oily film than that for HD (Fig. 7d) which is corroborated with friction and wear data.
Conclusions
A simple and cost-efficient method for the preparation of biodiesel from the WCO has been presented here. Triglycerides present in WCO were esterified via transesterification reaction to form fatty acid methyl ester (biodiesel) using a base catalyst. FT-IR, 1H-NMR, and 13C-NMR spectroscopy revealed that free carboxylic acid functionality successfully alters to methyl ester of the same hydrocarbon chain during the reaction. The fuel efficiency of the prepared biodiesel was assessed based on viscosity measurements, fire and flash point, TAN analysis, specific gravity, foaming stability, copper corrosion tendency, and emulsion analysis and compared with existing diesel fuel. All the tested parameters of BD were found within the ASTM limit. The BD showed higher flash and fire point than the existing diesel fuel, thereby revealed to have better fuel efficiency. The lubricating efficiency of the biodiesel was compared with hydrocarbon base oil hexadecane. It was found that the prepared BD lowered the COF up to 43% and specific wear rate ~ 71% compared with HD at an applied load of 30–80 N with rotating sliding velocity of 0.4 m/s in a macrotribometer. The polar-imparting oxygen atoms on biodiesel have better adherence property to the steel substrate, which can form a stable antifrictional transfer film into the rubbing tribo pairs, which effectively reduced the COF. Microtribological results with reciprocating sliding were also corroborated the above data. Prepared BD provided lower COF (~ 0.1–0.11) than HD (~ 0.12–0.14) with a negative wear depth under all loading conditions. The negative wear depth further supported the accumulation of palmitic ester chains during sliding and formed of a surface-active adhesive film, which was investigated by post tribological FE-SEM images of worn tribo tracks. Therefore, use of biodiesel can reduce fuel consumption due to its better lubricating property and can be a suitable alternative fuel and lubricant for automotive industries.
References
Karthickeyan V (2020) Experimental investigation on combined effect of ignition promoters and ceramic coating fuelled with papaya seed oil methyl ester in DI diesel engine. Renew Energy 148:772–789. https://doi.org/10.1016/j.renene.2019.10.163
Degfie TA, Mamo TT, Mekonnen YS (2019) Optimized biodiesel production from waste cooking oil (WCO) using calcium oxide (CaO) nanocatalyst. Sci Rep 9:18982. https://doi.org/10.1038/s41598-019-55403-4
Altin R, Cetinkaya S, Yucesu HS (2001) Potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers Manag 42:529–538. https://doi.org/10.1016/S0196-8904(00)00080-7
Gopal KN, Pal A, Sharma S, Samanchi C, Sathyanarayanan K, Elango T (2014) Investigation of emissions and combustion characteristics of a CI engine fueled with waste cooking oil methyl ester and diesel blends. Alex Eng J 53:281–287. https://doi.org/10.1016/j.aej.2014.02.003
Karthickeyan V (2019) Effect of combustion chamber bowl geometry modification on engine performance, combustion and emission characteristics of biodiesel fuelled diesel engine with its energy and exergy analysis. Energy 176:830–852. https://doi.org/10.1016/j.energy.2019.04.012
Nanthagopal K, Ashok B, Kishna RS, Srinath R, Kumar MP, Karthickeyan V (2020) Experimental investigation on engine parameters variation in common rail direct injection engine fueled with biodiesel. Clean Techn Environ Policy 22:459–479. https://doi.org/10.1007/s10098-019-01796-9
Canakci M, Gerpen JV (2001) Biodiesel production from oils and fats with high free fatty acids. Trans ASAE 44:1429–1436. https://doi.org/10.13031/2013.7010
Zahan KA, Kano M (2018) Biodiesel production from palm oil, its by-products, and mill effluent: a review. Energies 11:2132. https://doi.org/10.3390/en11082132
Carlinia M, Castelluccib S, Cocchia S (2014) A pilot-scale study of waste vegetable oil transesterification with alkaline and acidic catalysts. Energy Procedia 45:198–206. https://doi.org/10.1016/j.egypro.2014.01.022
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16:143–169. https://doi.org/10.1016/j.rser.2011.07.143
Barnwal BK, Sharma MP (2005) Prospects of biodiesel production from vegetable oils in India. Renew Sust Energ Rev 9:363–378. https://doi.org/10.1016/j.rser.2004.05.007
Zhang H, Ding J, Zhao Z (2012) Microwave assisted esterification of acidified oil from waste cooking oil by CERP/PES catalytic membrane for biodiesel production. Bioresour Technol 123:72–77. https://doi.org/10.1016/j.biortech.2012.06.082
Haigh KF, Vladisavljevic GT, Reynolds JC, Nagya Z, Saha B (2014) Kinetics of the pre-treatment of used cooking oil using Novozyme 435 for biodiesel production. Chem Eng Res Des 92:713–719. https://doi.org/10.1016/j.cherd.2014.01.006
Kulkarni MG, Dalai AK (2006) Waste cooking oils, an economical source of biodiesel: a review. Ind Eng Chem Res 45:2901–2913. https://doi.org/10.1021/ie0510526
Kawentar WA, Budiman A (2013) Synthesis of biodiesel from second-used cooking oil. Energy Procedia 32:190–199. https://doi.org/10.1016/j.egypro.2013.05.025
Karthickeyan V, Thiyagarajan S, Ashok B, Geo VE, Azad AK (2020) Experimental investigation of pomegranate oil methyl ester in ceramic coated engine at different operating condition in direct injection diesel engine with energy and exergy analysis. Energy Convers Manag 205:112334. https://doi.org/10.1016/j.enconman.2019.112334
Dabi M, Saha UK (2019) Application potential of vegetable oils as alternative to diesel fuels in compression ignition engines: a review. J Energy Inst 92:1710–1726. https://doi.org/10.1016/j.joei.2019.01.003
Kalam MA, Masjuki HH (2003) Effect of palm oil methyl ester and its emulsions on lubricant degradation and engine component wear. Lubr Sci 16:57–65. https://doi.org/10.1002/ls.3010160105
Alton R, Cetinkaya S, Yucesu HS (2001) The potential of using vegetable oil fuels as fuel for diesel engines. Energy Convers Manag 42:529–538. https://doi.org/10.1016/S0196-8904(00)00080-7
Karthickeyan V, Dhinesh B, Thiyagarajan S, Geo VE (2019) Investigating the combined effect of thermal barrier coating and antioxidants on pine oil in DI diesel engine. Environ Sci Pollut Res 26:15573–15599. https://doi.org/10.1007/s11356-019-04649-6
Karthickeyan V, Thiyagarajan S, Geo VE, Ashok B, Nanthagopal K, Chyuan OH, Vignesh R (2019) Simultaneous reduction of NOx and smoke emissions with low viscous biofuel in low heat rejection engine using selective catalytic reduction technique. Fuel 255:115854. https://doi.org/10.1016/j.fuel.2019.115854
Dhar A, Kevin R, Agarwal AK (2012) Production of biodiesel from high-FFA neem oil and its performance, emission and combustion characterization in a single cylinder DICI engine. Fuel Process Technol 97:118–129. https://doi.org/10.1016/j.fuproc.2012.01.012
Guru M, Koca A, Can O, Cinar C, Sahin F (2010) Biodiesel production from waste chicken fat based sources and evaluation with Mg based additive in a diesel engine. Renew Energy 35:637–643. https://doi.org/10.1016/j.renene.2009.08.011
Adewale P, Dumont MJ, Ngadi M (2015) Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew Sustain Energy Rev 45:574–588. https://doi.org/10.1016/j.rser.2015.02.039
Ferrero GO, Almeida MF, Ferraz MCMA, Dias JM (2015) Glycerol-enriched heterogeneous catalyst for biodiesel production from soybean oil and waste frying oil. Energy Convers Manag 89:665–671. https://doi.org/10.1016/j.enconman.2014.10.032
Mahesh SE, Ramanathan A, Meera KM, Begum S, Narayanan A (2015) Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energy Convers Manag 91:442–450. https://doi.org/10.1016/j.enconman.2014.12.031
Lee AF, Bennett JA, Manayil JC, Wilson K (2014) Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem Soc Rev 43:7887–7916. https://doi.org/10.1039/C4CS00189C
Ali OM, Mamat R, Abdullah NR, Abdullah AA (2016) Analysis of blended fuel properties and engine performance with palm biodiesel-diesel blended fuel. Renew Energy 86:59–67. https://doi.org/10.1016/j.renene.2015.07.103
Girotto F, Alibardi L, Cossu R (2015) Food waste generation and industrial uses: a review. Waste Manag 45:32–41. https://doi.org/10.1016/j.wasman.2015.06.008
Li M, Zheng Y, Chen Y, Zhu X (2014) Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour Technol 154:345–348. https://doi.org/10.1016/j.biortech.2013.12.070
Tsai WT, Lin CC, Yeh CW (2007) An analysis of biodiesel fuel from waste edible oil in Taiwan. Renew Sust Energ Rev 11:838–857. https://doi.org/10.1016/j.rser.2005.05.005
Reddy SM, Sharma N, Gupta N, Agarwal AK (2018) Effect of non-edible oil and its biodiesel on wear of fuel injection equipment components of a genset engine. Fuel 222:841–851. https://doi.org/10.1016/j.fuel.2018.02.132
Aboelazayem O, Gadalla M, Saha B (2018) Valorisation of high acid value waste cooking oil into biodiesel using supercritical methanolysis: experimental assessment and statistical optimisation on typical Egyptian feedstock. Energy 162:408–420. https://doi.org/10.1016/j.energy.2018.07.194
Samanta S, Singh S, Sahoo RR (2019) Effect of thermal annealing on the physico-chemical and tribological performance of hydrophobic alkylated graphene sheets. New J Chem 43:2624–2639. https://doi.org/10.1039/C8NJ05516E
Soares IP, Rezende TF, Silva RC, Castro EVR, Forte ICP (2008) Multivariate calibration by variable selection for blends of raw soybean oil/biodiesel from different sources using Fourier transform infrared spectroscopy (FT-IR) spectra data. Energy Fuel 22:2079–2083. https://doi.org/10.1021/ef700531n
Siatis NG, Kimbaris AC, Pappas CS, Tarantilis PA, Polissiou MG (2006) Improvement of biodiesel production based on the application of ultrasound: monitoring of the procedure by FTIR spectroscopy. J Am Oil Chem Soc 83:53–57. https://doi.org/10.1007/s11746-006-1175-1
Dube MA, Zheng S, Mclean DD, Kates MJA (2004) Comparison of attenuated total reflectance-FTIR spectroscopy and GPC for monitoring biodiesel production. J Am Oil Chem Soc 81:599–603. https://doi.org/10.1007/s11746-006-0948-x
Odetoye TE, Ogunniyi DS, Olatunji GA (2010) Preparation and evaluation of Jatropha curcas Linnaeus seed oil alkyd resins. Ind Crop Prod 32:225–230. https://doi.org/10.1016/j.indcrop.2010.04.016
Sarpal AS, Silva PRM, Silva SR, Monteiro TV, Itacolomy J, Cunha VS, Daroda RJ (2015) Direct method for the determination of iodine value of biodiesel by quantitative nuclear magnetic resonance (q1H-NMR) spectroscopy. Energy Fuel 29:7956–7968. https://doi.org/10.1021/acs.energyfuels.5b01462
Sarpal AS, Costa ICR, Teixeira CMLL, Filocomo D, Candido R, Silva PR, Cunha VS, Daroda RJ (2016) Investigation of biodiesel potential of biomasses of microalgae chlorella, spirulina and tetraselmis by NMR and GC-MS techniques. J Biotechnol Biomater 6:1–15. https://doi.org/10.4172/2155-952X.1000220
Sarpal AS, Silva PRM, Martins JL, Amaral JJ, Monnerat MM, Cunha VS, Daroda RJ, Souza WD (2014) Biodiesel potential of oleaginous yeast biomass by NMR spectroscopic techniques. Energy Fuel 28:3766–3777. https://doi.org/10.1021/ef402516x
Karthickeyan V (2018) Effect of thermal barrier coating on performance and emission characteristics of kapok oil methyl ester in diesel engine. Aust J Mech Eng:1–14. https://doi.org/10.1080/14484846.2018.1546450
Karthickeyan V (2019) Effect of cetane enhancer on Moringaoleifera biodiesel in a thermal coated direct injection diesel engine. Fuel 235:538–550. https://doi.org/10.1016/j.fuel.2018.08.030
Sahoo RR, Biswas SK (2009) Frictional response of fatty acids on steel. J Colloid Interface Sci 333:707–718. https://doi.org/10.1016/j.jcis.2009.01.046
Acknowledgments
Special thanks to Central Research Facility, CSIR-CMERI for providing the FE-SEM data and to the Director, CSIR-CMERI for permission to publish this work.
Funding
The authors received a grant through 12FYP project ESC-0112 to CSIR, India, in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
1H NMR, 13C NMR and optical images of the counterface ball wear scar have been provided as supporting information. (DOCX 1765 kb)
Rights and permissions
About this article
Cite this article
Samanta, S., Sahoo, R.R. Waste Cooking (Palm) Oil as an Economical Source of Biodiesel Production for Alternative Green Fuel and Efficient Lubricant. Bioenerg. Res. 14, 163–174 (2021). https://doi.org/10.1007/s12155-020-10162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10162-3