Abstract
This study demonstrated the performance of the sugarcane bagasse ash (SCBA) impregnated with calcium oxide (CaO) as a novel heterogeneous basic catalyst in biodiesel production. The SCBA was prepared by calcination for 2 h at 500 to 800 °C and impregnated with CaO loadings (10 to 40 wt.%). The prepared SCBA/CaO catalyst was characterized using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), temperature programmed desorption of carbon dioxide (TPD-CO2), thermal gravimetric analysis (TGA), X-ray fluorescence (XRF) and Brunauer-Emmett-Teller (BET) surface characteristics. A series of transesterification reactions were conducted to evaluate the performance of the catalysts. As a result, highest FAME yield of 93.8% was obtained by using SCBA600°C CaO(40%) catalyst at 20:1 methanol-to-oil molar ratio, reaction temperature of 65 °C, with 6 wt.% catalyst in 3 h. Besides, the catalyst can be reused up to 5 reaction cycles with biodiesel yield of 93.0% and 70.3% at first and fifth cycles, respectively. In this work, it was found that the natural SiO2 in the SCBA has a significant role to enhance the catalytic performance and reduce the catalyst’s deactivation drawback by minimizing the leaching of active sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In realization of world fuel crisis, many researchers are drawn in finding the new renewable energy source in order to displace the current fossil-based fuel; this scenario leads to the introduction of biodiesel. It was reported that, the easiest and cost-effective biodiesel production is by transesterification process [1]. The transesterification process can be catalyzed either by acidic or basic catalysts which may exist in homogeneous or heterogeneous forms. In term of the biodiesel production process, the heterogeneous type of catalyst is recommended over the homogeneous catalyst such as sodium hydroxide and potassium hydroxide due to fewer corrosive properties, easy separation and low environmental pollution [2].
The heterogeneous catalysts are further divided into two which are solid basic and also solid acid catalysts, where the solid basic catalyst is more preferable for the transesterification process [3]. This is due to some advantages possessed by solid basic catalyst, such as long catalyst life, high activity and only requires moderate conditions in order to operate [4]. CaO is one of the most studied solid basic catalysts due to its high basicity and also due to its ability, which can be derived from cheap and plenty natural resources such as waste eggshells, cockle shells and oyster shells [5]. However, Chen et al., [6] has reported that the CaO catalyst also holds some critical drawbacks, which when they realised that the leaching of Ca2+ ions from CaO surface decreases its catalytic activity after a few cycles. Moreover, an article by Marinković et al., [7] added that the reduced activity of CaO might be contributed from the poisoning of CaO by adsorption of CO2 and H2O from air exposure.
Therefore, the implementation of catalyst support becomes an option not only to improve the catalyst’s stability but also to enhance its performance. There are various types of catalyst support such as metal oxides which includes MgO, ZnO and Fe2O3 [8,9,10]. However, according to Wang et al. [11], the metal oxides as supports are not quite practical due to complex preparation technique, costly and suffer from deactivation. Hence, the non-metal oxides such as zeolites, alumina and silica supports are preferable alternatives, as it known as a good catalyst support due to its properties which includes good thermal stability, high surface area and also unique large pore characteristic [12,13,14]. Witoon et al. [15] have proved the applicability of silica as the catalyst support for Ca materials through a study using the Ca/Si composites for the transesterification process. To avoid the additional cost of commercialized silica, there have been alternative efforts to utilize agricultural wastes to obtain natural silica contents [14]. Wang et al. [11] have found that the waste peat able to serve as a support for the CaO and able to be reused up to 10 reaction cycles with excellent catalytic activity. They also reported that the formation of Ca–O–Si interaction between the natural silica content and CaO improved the catalyst stability and reduced the leaching of active sites.
Sugarcane bagasse ash (SCBA) is an abundantly available agricultural waste resulted from the burning process of sugarcane bagasse after the sugar extraction [16]. Compared to other biomass waste, this work anticipated to explore the catalytic role of SCBA due to its significant amount of natural silica content which might be applied as the catalyst support [16,17,18]. Moreover, due to its abundance which often causes the improper disposal problem, the utilization of the sugarcane bagasse ash waste will benefit in terms of the waste management and thus helps to reduce the environmental pollution.
To date, no implementation of SCBA as a catalyst support has been reported. Therefore, in this work, SCBA was used for the first time as a catalyst support for CaO to determine its feasibility as a novel basic heterogeneous catalyst for the biodiesel production. The optimum preparation conditions including the best calcination temperatures and catalyst loading were determined. Various characterization methods were implemented to study the morphological and chemical properties of the synthesized catalysts. The catalytic performance under various parameters together with the reusability analysis was also evaluated.
Materials and Methodology
Materials
Sugarcane bagasse (SCB) was collected from sugarcane juice industries in Shah Alam, Malaysia. On the other hand, the edible-grade palm oil was supplied from local market near Shah Alam, Selangor, Malaysia. The potassium hydroxide, KOH (99%); commercialized calcium oxide, CaO (99%); tetrahydrofuran, THF (99%); methanol, CH3OH of analytical grade (99.5%); and internal standard (methyl heptadecanoate (98%)) with FAME mix brand Supelco CRM1918 were purchased from Merck Aldrich chemical company. All reagents and solvents used in this work were of analytical grade and no purification was needed. The physiochemical properties of the palm oil were analysed using standard method based on American Society for Testing and Material (ASTM) standard and Malaysian palm oil board (MPOB), such as acid value (ASTM D974), FFA value (ASTM D974), saponification value (ASTM D974), molecular weight (ASTM D464) and moisture content (ASTM MPOB), and the results are presented in Table 1.
Preparation of SiO2-Rich SCBA/CaO Solid Basic Catalyst
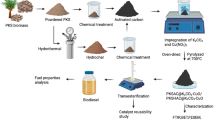
Briefly, SCB was sieved to 60–100 mesh and then washed with deionized water thoroughly in order to remove the unnecessary materials. The SCB was then dried at 105 °C to remove all moisture content, followed by calcination process at temperatures of 500 °C, 600 °C, 700 °C and 800 °C to obtain the optimum calcination temperature to acquire SCB ash (SCBA). The resulted ashes were then preceded to serve as the catalyst support and labelled as SCBA (500°C), SCBA (600°C), SCBA (700°C) and SCBA (800°C), respectively.
The catalyst activation method was adopted from Chen et al. [12] with slight modification. The prepared SCBA was then impregnated with different percentage of CaO (10%, 20%, 30% and 40%) using the wet impregnation method. To prepare 10% CaO loading on SCBA, typically 1 g of CaO was mixed with 50 mL of distilled water. The solution consisting Ca (OH)2 was then added with 9 g of SCBA and proceeded to continuous stirring at 400 rpm for 4 h and was left for 24 h for ageing process. The white precipitate was re-calcined at temperature of 600 °C for 2 h to produce SCBA/CaO catalyst.
Characterizations of Sugar Cane Bagasse Ash Catalyst
To ensure the successful preparation of the CaO supported on the SCBA, few characterization techniques were carried out. The Phenon XL scanning electron microscopy (SEM) instrument was employed to provide the morphological analysis of the prepared catalyst. The identification of the functional groups on the catalyst was recorded by using Fourier-transform infrared spectroscopy (FTIR) by Perkin-Elmer IR spectrometer ranging 400 to 4000 cm−1. Meanwhile, analysis using X’Pert Pro, Panalytical X-ray diffraction instrument was also implemented to determine the detailed qualitative aspect of the prepared catalyst. The catalyst stability towards the thermal exposure was obtained through SETARAM SETSYS Evolution 1750 instrument of thermal gravimetric analyser.
On the other hand, the quantity, distribution and strength of active sites were examined by Thermo Finnigan TPDRO 1100 series temperature programmed desorption of carbon dioxide (TPD-CO2). Moreover, the exact amount of CaO impregnated on the SCBA was quantified with Epsilon 3 XL Benchtop of X-ray fluorescence (XRF) spectrometer. Furthermore, the studies on the surface characteristics of SCBA and SCBA/CaO were accomplished by the physical absorption of gas molecules on solid surfaces including the pore volume and pore diameter by Belsorp II Brunauer-Emmett-Teller, N2 sorption analysis.
Transesterification of Palm Olein Oil
The catalytic activity of the prepared catalysts was evaluated via the transesterification of palm oil. The reaction was conducted in 150 mL of three-necked round-bottom flask connected with condenser in oil bath. Generally, an amount of catalyst, methanol and 30 g of palm olein oil were added in the reactor and heated up to 65 °C and stirred at 500 rpm. Catalyst was then separated from the products through centrifugation at the end of the reaction while the excess of methanol was recovered by rotary evaporation. The final product mixture was allowed to separate into two layers: biodiesel at the top and glycerol at the bottom. Biodiesel product was then collected and analysed by using Agilent 7890A Series Gas Chromatography with flame ionization detector (GC-FID) and equipped with HP-INNOWax polar capillary column with 30 m length and internal diameter of 0.5 mm. Carrier gas used was helium gas with flow rate of 1.5 mL min−1. The temperature programming employed for the analysis was setup as follows: initial oven temperature of 100 °C held for 1 min, continued with temperature ramping at the rate of 30 °C min−1 to 190 °C for 2 min and raised to 250 °C with rate of 10 °C min−1 and maintained for 7 min. Meanwhile, the injector and detector ports were set at 250 °C and 280 °C, respectively. Prior to the biodiesel analysis, the product was diluted with n-hexane containing methyl heptadecanoate as internal standard. The hydrogen and air intakes were set at 450 mL and 40 mL min−1, respectively. The biodiesel yield was finally computed using equation from standard method EN 14103.
Reusability of the Catalyst
The reusability of the catalyst was evaluated by reuse of the spent catalyst for the next reaction cycle. The spent catalyst was washed carefully with THF several times to remove the remaining methanol and reaction product on catalyst surface and reused without thermal activation. The biodiesel yield obtained from the product through each cycle was recorded.
Product Assessment by Chromatographic Analysis
The produced methyl ester’s compositions were further confirmed via Agilent Technologies 5890 Series II gas chromatography equipped with HP 5971 mass spectrometer detector and a 30 m × 250 μm × 0.25 μm of HP 5-MS capillary column. The temperature of injector and detector ports was programmed at 250 °C and 300 °C, respectively, with He as carrier gas (30 mL s−1). The oven was set initially at 60 °C and increased up to 170 °C with the heating rate of 10 °C min−1.
Results and Discussion
Preliminary Analysis of the Feedstock (Palm Oil)
Preliminary analysis of the feedstock is very important to design the catalyst suitable for the type of the feedstock. In this work, Table 1 shows the properties of the palm olein oil resulted from the test carried out using standard methods of ASTM and MPOB Malaysia. It was observed that the low acid content and high glyceride content indicated the compatibility for the alkaline types of the catalyst to catalyze this reaction [19].
Catalyst Characterization
It is well-established that the calcination process is an important step in the catalyst preparation process, not only to remove all unnecessary volatile compounds, but it also could significantly affect the structure and efficiency of the catalyst itself [20]. The SCBA was initially calcined at various temperatures and subjected to BET analysis to understand how calcination temperatures can affect the surface structure and porosity of the SCBA, as in Table 2. It can be learnt that the largest surface area of 2905.8 m2 g−1, pore volume of 22.716 cm3 g−1 and pore radius of 31.27 nm of SCBA were resulted from the calcination process at temperature of 500 °C. However, the surface area, pore volume and pore size of the SCBA were reduced as the calcination temperature increased to 600 °C, 700 °C and 800 °C. This can be explained by the sintering effect which could permanently damage the surface structure caused by high temperature during the thermal treatment [21]. Table 2 also lists the surface area of other biomass ashes from previous studies. Each biomass ash exhibits different surface area and pore sizes depending on the calcination condition. Interestingly, compared to other biomass ashes in the previous studies, the SCBA in this study presents the highest surface area.
Besides, the changes of the surface area and the porosity of the SCBA catalysts could also be associated with the amount of metal impregnation. The impregnation of the CaO on the SCBA catalyst’s support was successfully done by wet impregnation process. It was found that the surface area, pore volume and pore size of SCBA impregnated CaO was significantly reduced from 2351.8 m2 g−1 to less than 6 m2 g−1. As shown in Table 2, as the amount of impregnated CaO increased, the surface area of the SCBA/CaO catalyst was declining from 5.37 to 4.18 m2 g−1 and the pore volume reduced from 62.096 to 40.712 cm3 g−1. This trend may be attributed by the distribution of CaO on the SCBA surfaces together with the clogging of available pores by CaO, and this continued to be more significant as the amount of the CaO impregnated increased [11]. However, when the CaO impregnation was increased up to 40 wt.%, no obvious enhancement to the CaO amount compared to 30 wt.% of CaO loadings was from 29.46 to 29.96 wt.%. This could probably be due to that SCBA medium was reaching its capacity limit and further addition of CaO loading beyond 40 wt.% might be too excessive.
Basically, different calcination temperature could lead to different degree of decarbonization process and affect the diffraction patterns of the catalyst structure. The XRD patterns for each SCBA are demonstrated in Fig. 1a. As received, the calcined SCBA at 500 °C exhibited several diffraction peaks at 2θ = 22.0°, 32.1°, 35.0°, 40.1° and 44.5° corresponded to the presence of SiO2 (JCPDS 01-082-1555). Meanwhile, diffraction peaks at 2θ = 27.6° and 29.0° were detected that matched to the K2O compound (JCPDS 00-023-0493). As the calcination temperatures were elevated further, the intensity of these peaks increased, showing that higher calcination temperature increases the metal oxide compositions [25].
On the other hand, Fig. 1b presents the XRD patterns of the SCBA that were calcined at fixed temperature of 600 °C but impregnated with varied CaO percentages from 10 to 40 wt.%. As demonstrated, when an amount of CaO was impregnated into SCBA, a few changes can be observed towards the XRD patterns. As more weight percent of CaO was introduced into the SCBA, a sharp reduction of SiO2 peaks intensity can be seen together with the appearance of CaO peaks at 2θ = 28°, 32° and 46° (JCPDS 00-021-0155). The XRD patterns of SCBA/CaO(40%) present the new peaks at 2θ = 35°and also 37° which were belonged to the characteristic peaks of Ca2SiO4 phase (JCPDS 01-083-0461). This presence of new peaks showed that there was a chemical reaction between SiO2 compound in the SCBA itself with the impregnated CaO forming new phase Ca2SiO4, and this interaction can help to improve the stability of the CaO catalyst by holding the Ca element through the Si–O–Ca bond [11].
The FTIR spectra of the SCBA, CaO and the SCBA/CaO(40%) catalysts are presented in Fig. 2. The presence of silica in the SCBA was further confirmed via IR spectra of SCBA which displayed major absorption band at 1026 cm−1 corresponded to the asymmetric stretching of Si–O–Si. Thus, it indicates the presence of siloxane bond [1]. This result was matched with the XRD pattern of raw SCBA that mainly contained with SiO2.
Meanwhile, according to the IR absorption band for SCBA40%CaO catalyst, the incorporation of CaO into the SCBA caused a new major band that appeared at 946.31 cm−1. This band was devoted to Si–O–Ca bond which further proved the existence of the interaction between CaO and SiO2 that contributed to the formation of a new compound namely Ca2SiO4 or CaSiO3 [6]. The introduction of CaO into SCBA also reduced the intensity and caused the weakening of the silica peak in IR spectrum at 1026 cm−1 (Si–O–Si), which was consistent to the previous XRD pattern. Meanwhile, the major absorption band at 3641 cm−1 and 871.67 cm−1 was signified to the stretching of O–H, and C–O bonds of CaO that were introduced into SCBA. The absorption band at region 1418 cm−1 indicated the presence of CO3−2 (carbonate ions) on SCBA40%CaO catalyst which is also due to the presence of CaO [14].
The surface morphologies of the SCBA catalysts are presented by SEM micrographs in Fig. 3. The irregular agglomeration can be observed for the structure of SCBA in Fig. 3a, which may be due to the formation of metal oxides after calcination process and also the formation of some network of pores [26]. Further impregnation of SCBA with 10 wt.% of CaO (Fig. 3b) showing the irregular surface structure with unclear pores indicated the presence of CaO that covered the surface and pores of SCBA. The morphological changes towards the raw SCBA were in line with the surface characteristics analysis (BET) where the increase in number of CaO on the SCBA reduced the surface area, pore volume and pore size of the catalyst.
Meanwhile, the EDS analysis of the SCBA, SCBA/CaO(10%), SCBA/CaO(20%), SCBA/CaO(30%) and SCBA/CaO(40%) catalysts revealed the existing components in the catalysts as shown in Table 3. It was observed that the major components of SCBA itself were composed of Si, Ca, K, P and Mg which contributed to the basicity of the catalyst. The ascending weight percentages of Ca elements in the SCBA after the impregnation of CaO confirmed that the process was done accordingly.
Figure 4 a shows the TPD-CO2 profile of the SCBA calcined at fixed 600 °C temperature with various CaO loadings. Before impregnation with any CaO, it was found that no major peaks can be observed for the SCBA that indicated that it has a quite low basic property. However, the basic sites densities were increased consistently with the addition of the CaO on the SCBA. The SCBA with 40 wt.% CaO catalyst possessed the highest basic densities with 38,895.75 μmol g−1 followed by 30 wt.% CaO, 20% wt. CaO and 10 wt.% CaO which were 2861.70, 2502.30 and 2119.20 μmol g−1, respectively, as shown in Table 2. The TPD-CO2 plots of 40 wt.% depicted the most intense peak at temperature between 600 °C and 700 °C when compared to lower CaO percentage catalysts, and the desorption peaks at the temperature zone more than 500 °C were associated with the strongly basic property [19].
Meanwhile, the calcination temperature also has a significant effect in determining the basic densities of the catalyst. Figure 4 b presents the basic densities of the catalyst with varied SCBA calcination temperatures with fixed CaO loadings at 40%. It was found that, the basic density of prepared catalyst was increased from 2895.19 to 3889.75 μmol g−1 when the calcination temperatures were increased from 500 to 600 °C. However, further increase of calcination temperature reduces the quantity of basic sites around 2861.20 to 3107.73 μmol g−1. This might be related to the surface area of the SCBA, which was lowered at high calcination temperature and hence reduced the amount of CaO on the surface of SCBA.
Out of the catalysts prepared, SCBA600°C CaO(40%) was then subjected to the TGA-DSC analysis to examine its thermal profile (supplementary data 1). The prepared catalyst demonstrated no major weight loss even at a high temperature around 500 °C with only 10% weight loss from the initial weight around 100 to 300 °C which was normally due to the removal of volatile compound and moisture [27]. As no critical weight changes were observed with the thermal profile of SCBA600°CaO (40%) catalyst, calcination temperature of 600 °C was considered enough to produce a thermally stable catalyst and higher temperature might be causing the unnecessary energy cost in catalyst production.
Effect of Calcination Temperature and Active Sites on the Activity of Catalyst
Basically, optimum calcination temperature of the SCBA is very important for industrialization to reduce the preparation cost of the catalyst without ignoring their performance. The SiO2-rich SCBA produced from various calcination temperatures were initially impregnated with 40 wt.% of CaO and subjected to the transesterification test for catalytic evaluation.
Figure 5 illustrates the relation between the calcination temperatures of SCBA with the biodiesel yield obtained. It was observed that the highest biodiesel yield obtained was 90% resulted from reaction catalysed by SCBA calcined at 600 °C. However, the biodiesel yield was notably descending as the calcination temperature of SCBA was raised up to 800 °C. This may be associated by the sintering effect which produced low surface area (Table 2), when the sample was heated above 600 °C. The damage of the catalyst structure will affect the performance of the catalyst itself by lowering its catalytic capability thus resulting in lower biodiesel yield [21]. Most importantly, the basicity or the active site distributions play a major role in determining the performance of the catalyst [28]. In relation, as obtained from Fig.4b, the catalyst prepared from the calcination temperature of 600 °C possessed the most basic densities which explain the high yield obtained when compared to catalysts of other calcination temperatures.
Meanwhile, Fig. 6 shows the effect of different loading of active sites on the SCBA towards the activity of prepared catalyst. It was found that the SCBA incorporated with 40 wt.% of CaO yielded the highest biodiesel yield which was 89.78% when compared to the other SCBA/CaO.
This may be attributed to the high basic densities of the catalyst. Even though the surface area of the SCBA impregnated with 40 wt.% was lowest from the previous BET analysis, it was counterpart by the high basic densities as characterized from previous TPD-CO2. Hence, the SCBA600°C CaO(40%) catalyst was chosen for further evaluation and used for determination of optimum parameter during transesterification process.
Effect of Reaction Parameters on Transesterification of Palm Olein Oil
For the optimization purpose, the SCBA600°C CaO(40%) catalyst was then subjected into a series of transesterification process with one varied reaction parameter at a time to obtain the final optimum reaction conditions. The parameters that were under study include the methanol–oil molar ratio, the catalyst loading, reaction temperature and reaction time.
For the methanol–oil molar ratio, the ratios were varied from 9:1 to 23:1 M ratio. Figure 7 a presents the correlation between the methanol–oil molar ratio towards the FAME yield or biodiesel yield assisted by 5 wt.% of catalyst loadings, 3 h of reaction time at 65 °C. It was observed that the FAME yield was in conjunction with the increment of methanol–oil molar ratio. Since, stoichiometrically a transesterification reaction requires 3 mol of methanol for each triglyceride and excess methanol amount helps to drive the reaction equilibrium towards the FAME product following the Le’Chateliers effect. However, the improvement of the FAME yield found to peak up to 91.2% at 20:1 methanol–oil molar ratio and declined as amount of methanol increased to 23:1 with slight decrease from 91.2 to 89% due to the occurrence of catalyst dilution by the excess methanol amount [11]. Meanwhile, the amount of catalyst also affects the rate of transesterification process. As regards, a series of the transesterification reactions were carried out under the reaction conditions of reaction temperature of 65 °C, methanol–oil molar ratio of 20:1 and 3 h as shown in Fig. 7b with varied catalyst amount. Initially, FAME yield rose up sharply when the catalyst amount is added from 4 to 5 wt.% yielding 53.9 to 89.36% of FAME yield. According to Chen et al. [29], this may be due to the additional amount of catalyst aid the contact between the reactant and catalyst which could produce higher conversion. The maximum of FAME yield can be seen up to 92% by addition of 6 wt.% catalyst. However, further addition of catalyst more than 6 wt.% only disturbs the reaction process. As been reported by Fadhil et al. [30], too much catalyst presence could increase the mass transfer resistance thus lowering the reactant concentration on catalyst surface, hence lowering the rate of reaction.
Figure 7 c shows the FAME yield response towards the reaction time from 1 to 5 h at fixed 65 °C reaction temperature, 20:1 methanol–oil molar ratio and 6 wt% of SCBA600°C CaO(40%) catalyst. The FAME yield recorded highest after 3 h reaction time which was 93.6% and the elongated reaction duration results in lower FAME yield. The circumstances could be influenced by the fact that too long reaction time will enhance the solubility of biodiesel in the glycerol thus reducing the FAME yield as has been stated by Chen et al. [12]. In addition, Wan et al. [31] reported that the elongation time could cause the reaction to shift backwards which is consequent to product loss.
Lastly, Fig. 7d displays the relation between the reaction temperatures and FAME yield. The reaction conditions were equally setup to 20:1 methanol–oil ratio, 3-h reaction time and 6 wt.% catalysts with varied temperatures (50 to 70 °C). The FAME yield was found to escalate from 65 to 93.8% as the reaction temperatures were elevated from 50 to 65 °C. Theoretically, increased temperature helps to boost the collision between the reactants hence increasing the rates of reactions due to the endothermic reaction, where the transesterification process needs energy to collide and react [32,33,34]. At the same time, the higher temperature also helps to lessen the limit of diffusion between the catalyst and reactants [35]. When the reaction was conducted over 70 °C, a small reduction of FAME yield was noted from 93.8 to 91% and this was related to methanol boiling point, where higher temperature forcing the methanol to rapidly evaporate obstructs the reaction between methanol, oil and catalyst [36]. Therefore, the reaction temperature was concluded to be optimum at 65 °C.
Catalyst’s Reusability Evaluation
The important part to commercialize the heterogeneous catalyst in biodiesel production is the ability to be easily separated and recyclable. In this work, the reusability test of the SiO2-rich SCBA/CaO was conducted and compared with the unsupported commercialized CaO catalyst as shown in Fig. 8. The SCBA600°CaO (40%) was remarked to perform higher catalytic activity than the unsupported commercial CaO, where the yield obtained was 92.36% which was higher than commercialized CaO at only 81.4%. This scenario may be linked to the higher basic properties resulted from the combination of SCBA and CaO compared to the CaO itself as referred to previous Fig.4a. The amount of existing basic sites in the SCBA that naturally exists including Ca, Si, P and Mg as reported in Table 3 might contribute to the enhanced basicity of the SCBA/CaO catalyst. In the stability aspect, the synthesized SCBA600°CaO (40%) catalyst was able to maintain its performance up to the 5th reaction cycle with acceptable biodiesel yield of 70.3%. The TPD-CO2 analysis showed that the basicity of the catalyst drops from 3895.75 to 2010.6789 μmol/g (supplementary data 2) after 5 reaction cycles which explains the reduced catalytic activity after the fifth cycle.
However, the unsupported CaO catalyst was found to have failed to accomplish a good yield after the 3rd cycle which was only below 50% conversion. According to Buasri et al. [37], poor performance of CaO-based catalyst after a series of consecutive cycles was a consequence from the bond breaking and formation of Ca(OH)2 or maybe calcium diglyceroxide on the catalyst surface and thus prohibited the interaction between the active sites of the catalyst and the reactants. Obviously, the synthesized catalyst (SCBA600°CaO (40%)) showed better stability than the unsupported commercial CaO, which was coherent to the existence of the CaO–SiO2 interaction, that successfully helps to minimize the lixiviation of calcium from catalyst surface [11, 14, 38].
The performance and stability of the prepared catalyst in this work was in-line with other related waste–ash catalyst summarized in Table 4. Lani et al. [14] and Ho et al. [26] produced CaO supported on palm mill and rice husk ashes producing 97% and 87% FAME yield, with 3 and 6 reaction cycles respectively.
Biodiesel Product Analysis
The biodiesel product obtained after the optimized reaction conditions was then preceded to be analysed by using GC-MS to confirm the methyl ester content in the FAME product and to obtain exact percentages by matching with the mass library spectral. The methyl ester percentages in the final product were justified in Table 5 below, where the produced biodiesel was confirmed to contain methyl esters that majorly consist of palmitic acid and oleic acid methyl esters with 36.43 and 53.14%, respectively, followed by myristic acid.
Conclusions
In overall view, this study was successfully evaluated the feasibility of the SiO2-rich SCBA/CaO catalyst as a novel heterogeneous catalyst in biodiesel production. The as-prepared catalyst exhibited a good catalytic performance when synthesized through the ideal preparation conditions which were 600 °C calcination temperature followed by 40 wt.% of CaO and operated under the optimum reaction conditions of 20:1 methanol oil ratio, 3 h reaction time, 6 wt.% catalyst loadings and 65 °C reaction temperature. The performance of the synthesized catalyst was superior than the unsupported commercial CaO itself when the maximum biodiesel yield obtained was 93.8% with five times recyclability while the commercial CaO only was able to achieve only 81.4% with three times recyclability. Most importantly, this study has explored and revealed the viability or the potential of natural SiO2-rich SCBA/CaO as novel and low-cost heterogeneous catalyst in biodiesel production.
References
Atadashi IM, Aroua MK, Aziz A, Sulaiman NMN (2012) Production of biodiesel using high free fatty acid feedstocks. Renew Sust Energ Rev 16(5):3275–3285
Singh V, Sharma YC (2017) Low cost guinea fowl bone derived recyclable heterogeneous catalyst for microwave assisted transesterification of Annona squamosa L. seed oil. Energ Convers Manage 138:627–637
Boey P-L, Pragas G, Hamid MS (2011) Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: a review. Chem Eng J 168(1):15–22
Math MC, Kumar SP, Chetty S (2010) Technologies for biodiesel production from used cooking oil – a review. Energy Sustain Dev 14(4):339–345
Maneerung T, Dai Y, Kawi S, Wang C-H (2016) Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energ Convers Manage 123:487–497
Chen G, Shan R, Li S et al (2015) A biomimetic silicification approach to synthesize CaO-SiO2 catalyst for the transesterification of palm oil into biodiesel. Fuel 153:48–55
Marinković DM, Stanković MV, Veličković AV et al (2016) Calcium oxide as a promising heterogeneous catalyst for biodiesel production: current state and perspectives. Renew Sust Energ Rev 56:1387–1408
Ezzah M, Lokman M, Saiman MI, HinTaufiq-Yap Y (2016) Synthesis and characterization of Fe2O3/CaO derived from Anadara Granosa for methyl ester production. Energ Convers Manage 126:124–131
Sudsakorn K, Saiwuttikul S, Palitsakun S, Seubsai A, Limtrakul J (2017) Biodiesel production from Jatropha Curcas oil using strontium-doped CaO/MgO catalyst. J Environ Chem Eng 5:2845–2852
Kesica Z, Lukic I, Zdujic M, Liu H, Skala D (2012) Mechanochemically synthesized CaO ZnO catalyst for biodiesel production. Procedia Eng 42:1169–1178
Wang S, Shanab R, Wang Y, Lu L, Yuan H (2019) Synthesis of calcium materials in biochar matrix as a highly stable catalyst for biodiesel production. Renew Energy 130:41–49
Chen GY, Shan R, Shi J-F, Yan B-B (2015) Transesterification of palm oil to biodiesel using rice husk ash-based catalysts. Fuel Process Technol 133:8–13
Melero JA, Bautista LF, Iglesias J, Morales G, Sánchez-Vázquez R (2012) Zr-SBA-15 acid catalyst: optimization of the synthesis and reaction conditions for biodiesel production from low-grade oils and fats. Catal Today 195(1):44–53
Lani NS, Ngadi N, Yahya NY, Rahman RA (2017) Synthesis, characterization and performance of silica impregnated calcium oxide as heterogeneous catalyst in biodiesel production. J Clean Prod 146:116–124
Witoon T, Bumrungsalee S, Vathavanichkul P, Palitsakun S, Saisriyoota M, Faungnawakij K (2014) Biodiesel production from transesterification of palm oil with methanol over CaO supported on bimodal meso-macroporous silica catalyst. Bioresour Technol 156:329–333
Falk G, Shinhe GP, Teixeira LB, Moraes EG, Oliveira AN (2019) Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions. Ceram Int 45:21618–21624
Faria KC, Holanda JN (2012) Using SEM/EDS for characterization of clay ceramic bearing sugarcane. In: Mendez-Vilas A (ed) Current microscopy contributions to advances in science and technology, vol. 2. Formatex, Badajoz, pp 1085–1092
Souza A, Teixeira S, Santos G, Costa F, Longo E (2011) Reuse of sugarcane bagasse ash (SCBA) to produce ceramic materials. J Environ Manage 92:2774–2780
Mansir N, Yap YH, Rashid U, Lokman MI (2017) Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: a review. Energ Convers Manage 141:171–182
Feyzi M, Shahbazi Z (2017) Preparation, kinetic and thermodynamic studies of Al–Sr nanocatalysts for biodiesel production. J Taiwan Inst Chem Eng 71:145–155
Zhao C, Lv P, Yang L, Xing S, Luo W, Wang Z (2018) Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energ Convers Manage 160:477–485
Freitas JV, Ruotolo LAM, Farinas CS (2019) Adsorption of inhibitors using a CO2-activated sugarcane bagasse fly ash for improving enzymatic hydrolysis and alcoholic fermentation in biorefineries. Fuel 251:1–9
Hindryawati N, Maniam GP, Karima MR, Chong KF (2014) Transesterification of used cooking oil over alkali metal (Li, Na, K) supported rice husk silica as potential solid base catalyst. JESTECH 17(2):95–103
Lee J, Jong-MinJung J-IO, Ok YS, Lee S-R, Kwon E, E. l. (2017) Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour Technol 231:59–64
Dai YM, Chen KT, Wang PH, Chen CC (2016) Solid-base catalysts for biodiesel production by using silica in agricultural wastes and lithium carbonate. Adv Powder Technol 27(6):2432–2438
Ho WW, Ng HK, Gan S, Tan SH (2014) Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energ Convers Manage 8:1167–1178
Roschat W, Siritanon T, Yoosuk B, Promarak V (2016) Rice husk-derived sodium silicate as a highly efficient and low-cost basic heterogeneous catalyst for biodiesel production. Energ Convers Manage 119:453–462
Faba EM, Ferrero OG, Dias MJ, Eimer AG (2018) Thermo-chemically tuning of active basic sites on nanoarchitectured silica for biodiesel production. Molecular Catalysis [In press]. Retrieved from https://doi.org/10.1016/j.mcat.2018.08.013
Chen K-T, Wang J-X, Dai Y-M, Wang P-H, Liou C-Y, Nien C-W et al (2013) Rice husk ash as a catalyst precursor for biodiesel production. J Taiwan Inst Chem Eng 44(4):622–629
Fadhil AB, Aziz AM, Al-Tamer MH (2016) Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energ Convers Manage 108:255–265
Wan L, Liu H, Skala D (2014) Biodiesel production from soybean oil in subcritical methanol using MnCO3/ZnO as catalyst. Appl Catal B Environ 152-153:352–359
Syazwani ON, Ibrahim ML, Wahyudiono K, Goto M, Taufiq-Yap YH (2017) Esterification of high free fatty acids in supercritical methanol using sulphated angel wing shells as catalyst. J Supercrit Fluids 124:1–9
Lokman IM, Rashid U, Taufiq-Yap YH (2015) Microwave-assisted methyl ester production from palm fatty acid distillate over a heterogeneous carbon-based solid acid catalyst. Chem Eng Technol 38(10):1837–1844
Farabi MSA, Ibrahim ML, Rashid U, Taufiq-Yap YH (2019) Esterification of palm fatty acid distillate using sulfonated carbon-based catalyst derived from palm kernel shell and bamboo. Energ Convers Manage 181:562–570
Gupta AR, Yadav SV, Rathod VK (2015) Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 158:800–806
Tan YH, Omar Abdullah M, Nolasco-Hipolito C, Taufiq-Yap YH (2015) Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: catalyst characterization and biodiesel yield performance. Appl Energy 160:58–70
Buasri A, Chaiyut N, Loryuenyong V, Wongweang C, Khamsrisuk S (2013) Application of eggshell wastes as a heterogeneous catalyst for biodiesel production. Sustain Energy 1(2):7–13
Jain D, Rani A, Khatri C (2010) Fly ash supported calcium oxide as recyclable solid base catalyst for Knoevenagel condensation reaction. Fuel Process Technol 91(9):1015–1021
Acknowledgements
The author would like to express the acknowledgements to the Ministry of Education (MOE) and Universiti Teknologi MARA for the research fund FRGS-RACER research grant 600-IRMI/ FRGS-RACER 5/3 (039/2019) and BESTARI research grant 600-IRMI/PERDANA 5/3 BESTARI (088/2018). Special thanks to Institute of Science (IOS) Universiti Teknologi MARA and Catalysis Science & Technology Research Centre, Universiti Putra Malaysia for all the facilities provided throughout this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. The success preparation of the CaO impregnated SiO2-rich SCBA as the heterogenous solid basic catalyst.
2. The prepared SCBA/CaO catalyst resulted 93.8% of FAME yield at mild reaction conditions for 3 h of transesterification process.
3. The SiO2 improves the reusability of catalyst up to 5 reaction cycles by improving the strength of the active sites-support bonding.
Electronic Supplementary Material
ESM 1
(DOCX 3155 kb)
Rights and permissions
About this article
Cite this article
Abdul Mutalib, A.A., Ibrahim, M.L., Matmin, J. et al. SiO2-Rich Sugar Cane Bagasse Ash Catalyst for Transesterification of Palm Oil. Bioenerg. Res. 13, 986–997 (2020). https://doi.org/10.1007/s12155-020-10119-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10119-6