Abstract
Supercritical water gasification (SCWG) of empty palm fruit bunches (EFBs) was investigated using ZnO-doped Ni–CaO catalysts for hydrogen-rich product gas. The catalysts were prepared via wet impregnation technique and characterized using XRD, BET, TPR–H2, and TPD–CO2. SCWG reactions were carried out 0.3 g of EFBs added to 5 wt% of the catalyst in 8 mL of deionized at 380 °C and the product gases were analyzed using gas chromatography. Incorporation of ZnO and Ni into CaO was found to be very active in promoting the water gas shift (WGS). From the various concentrations of dopants, 5 wt% ZnO with 5 wt% Ni was found to be the optimum loading on CaO, showing the highest hydrogen production (105.7 mmol mL−1). Besides, the formation of a Ni.8Zn.2O phase from the strong interaction between the dopants was found to be an active phase in promoting the WGS reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Supercritical water gasification (SCWG) is gaining notable attention from researchers as a promising method for converting biomass into hydrogen-rich product gases. The technique is considered economical in terms of low reaction temperatures and the fact that it can tolerate high moisture contents in the feedstock, where drying is a must for current conventional thermochemical methods in use (gasification, pyrolysis, and combustion). The alternative SCWG method will have a broad application in the near future if the biomass contains significant amounts of moisture, which would normally require prior drying before further upgrading processes.
Lignocellulose constitutes a major part of the cell wall of terrestrial plants and covers approximately 90% of accessible biomass. Tapping these huge resources in the form of agricultural, forestall, industrial residues, and wastes for second-generation biofuel is considered additional advantage where it is not in direct competition with food and feed production [1]. Palm oil industry accountable for 85.5% total biomass wastes produced in Malaysia with an average of 50 million tons dry oil palm residues annually and predicted to reach 100 million tons by 2020 [2, 3]. Among the palm oil mill wastes, empty fruit bunches (EFBs) are considered a potential feedstock for the SCWG process, due to its high moisture content and abundance, which causes waste-management problems. Even though many plausible ways have been implemented to deal with this discarded mill waste, including utilizing it as an organic fertilizer and boiler fuel, it is still not fully converted into valuable end products. Thus, EFB utilization as a SCWG feedstock is very promising and could initiate further studies in the near future on a larger scale.
The reaction temperature and the catalysts employed are the most vital factors in the SCWG that significantly impact the overall process efficiency and desired product yield. The reaction temperature of the SCWG has been classified into two main categories: low temperature (350–600 °C), which requires catalyst addition, and high temperature (500–750 °C), which is performed in the absence of a catalyst or with a nonmetallic catalyst [4]. The types of catalysts used in SCWG reactions are categorized into heterogeneous and homogenous, based on the phase of the catalyst and the reactant. Generally, homogenous catalysts include alkaline-metal catalysts such as Na2CO3, K2CO3, KHCO3, NaOH, KOH, and Ca(OH)2, which are widely reported; these catalysts dissolve in water and are capable of enhancing water gas shift (WGS) reactions [5,6,7,8,9]. However, catalyst recovery is difficult to accomplish from the reaction phase and continued additions without recycling are expensive [5, 10]. Thus, more attention is given to the development of heterogeneous catalysts in order to achieve similar catalytic activity and high selectivity towards hydrogen production.
Numerous types of heterogeneous catalysts have been investigated to accelerate the SCWG reaction, and Azadi et al. [11] classified the catalysts into three major categories: activated carbon (AC), transition metals (supported and unsupported), and oxides. AC catalysts have been reported to actively improve WGS reactions and methanation, and almost complete carbon conversion can be achieved at reaction temperatures around 600 °C [12]. However, at lower reaction temperatures, carbon-based catalysts have been found to be inactive in improving the rate of gasification [13]. Thus, the choice of the catalyst has been expended to a wide range of transition metals that are divided into supported and unsupported catalysts. Among the transition metals, nickel and ruthenium are widely used, owing to a promotional effect on hydrogen yield and carbon gasification [13,14,15]. In addition, several types of oxides have also been studied in the SCWG reaction and have shown significant improvements in hydrogen yield. Cao et al. studied the catalytic properties of 12 types of transition metal oxides (V2O5, Cr2O3, MnO2, Fe2O3, Co2O3, CuO, ZnO, ZrO2, MoO3, SnO2, WO3, and CeO2) in SCWG and concluded that most of the metal oxides significantly improve the gasification efficiency and also influence the overall product gas composition [16]. Besides, CaO also has been reported to catalyze gasification reactions, hydrocarbon-reforming reactions, and WGS reactions [17]. Thus, in this investigation, CaO-based catalysts were synthesized with the addition of suitable dopants (Ni and Zn) to enhance the gasification and hydrogen-favored reaction at moderate temperature (380 °C) using EFBs as a feedstock. Based on our previous studies, the addition of Zn into Ni-doped CaO catalysts significantly improves the hydrogen yield, owing to the major promotional effect on the WGS reaction. Thus, an optimization of Zn and Ni addition into CaO catalysts was carried out and the impact on EFB gasification to produce hydrogen-rich product gas is reported.
Experimental Section
Raw Materials and Reactants
The EFB feedstock used in this study was obtained from a local palm oil processing mill at Johor, Malaysia. The raw bunch was cut into smaller pieces and dried for 2 weeks. The dried sample was ground into powder (250 μm). The weight percentages of C, H, and O in the feedstock were assessed using elemental analysis and are listed in Table 1. The chemicals that were used in the preparation of the catalysts were CaO (24856-8), Ni(NO3)2·6H2O (244074), and Zn(NO3)2·6H2O (228737), all purchased from Sigma Aldrich.
Catalyst Preparation
Ni- and Zn-doped CaO catalysts were prepared in a single-step wet impregnation method. The addition of dopants was fixed in the order of 5% Ni with 5, 10, and 15 wt% Zn as well as 5% Zn with 5, 8, 10, 12, and 15 wt% Ni on CaO. The aqueous solutions of metal nitrates containing pre-defined ratios were added to bulk CaO and stirred for 6 h at ambient temperature. Then, the slurries were dried in an oven at 110 °C overnight and the obtained solids were crushed into powders. The powdered catalyst precursors were calcined in air at 900 °C with a heating rate of 20 °C min−1 for 6 h. Then, the obtained catalysts were crushed into a fine powder and reduced at 700 °C in a partial hydrogen flow (5% H2-balanced Ar) 20 mL min−1 for 3 h. The prepared catalysts are known as 5Zn5Ni–CaO, 10Zn5Ni–CaO, 15Zn5Ni–CaO, 5Zn8Ni–CaO, 5Zn10Ni–CaO, 5Zn12Ni–CaO, and 5Zn15Ni–CaO, which were kept in a vacuum desiccator before the catalytic reactions.
Characterization
The physicochemical properties of the catalysts were characterized by X-ray diffraction (XRD), Brunner–Emmet–Teller (BET) surface area measurements, and temperature-programmed reduction (TPR). The XRD patterns of the catalysts were analyzed at ambient temperature using a Shimadzu diffractometer (model XRD6000) equipped with a Philips glass diffraction X-ray tube of broad focus 2.7 kW type to produce Cu Ka radiation. The BET surface areas of the catalysts were obtained using a Thermo Finnigan Sorptomatic 1900 series instrument via N2 adsorption/desorption isotherms patterns at − 196 °C. TPR analysis was performed using Thermo Finnigan TPDRO 1100 apparatus equipped with a thermal conductivity detector. Each sample (20 mg) was pretreated in a N2 environment and continued analysis was performed in a 5%H2/argon flow (flow rate = 25 mL min−1) in the temperature range 50–900 °C. TPD–CO2 measurements performed with catalyst (20 mg) pretreatments at 400 °C in a N2 flow were subjected to CO2 gas adsorption at 50 °C for 1 h. Raising the catalyst temperature releases the adsorbates (CO2) in the presence of a carrier gas (He), which are detected by a thermal conductivity detector (TCD).
Catalytic Reaction
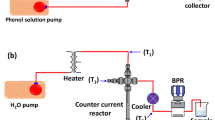
A custom-made reactor system was designed (Fig. 1) using stainless-steel tubing (SS-810-6-2, outside diameter (OD) = 1/8 in) connected with one-way valves. SCWG reactions were carried out in 13-mL reactor cells made from stainless-steel tubing (SS-t8-049-6ME) with OD = 1/2 in. A Swagelok transducer (limitation 0–400 atm) was used to measure the pressure of the reactor cell during the reaction. The catalytic tests were performed using 0.3 g of the feedstock (EFB) and 5 wt% of a catalyst loaded into the reactor cell, followed by 8 mL of deionized water. The catalytic tests were replicated 3 times. A gas chromatograph (GC) oven was used as the heating chamber; the reactor cell was placed inside and the temperature was increased from 30 to 380 °C with a ramping rate of 10 °C min−1. Then, the reaction time was fixed at 8 min. The reactor was cooled after the completion of each reaction, and both valves were opened intermittently to release the product gas, which was then trapped and collected using a 1-mL Luer lock gas-tight syringe. The gaseous products were injected into a GC with a thermal conductivity detector (GC-TCD) equipped with Porapak Q and Mole Sieve columns for CO2, H2, CH4, and CO detection.
Results and Discussion
Characterization
Crystalline structures of the calcined catalysts after reduction were examined using XRD, and the patterns are shown in Fig. 2. The obtained catalyst diffraction patterns exhibit three distinctive peaks relative to the presence of CaO at 32.4, 37.5, and 54°. In addition, several Ca(OH)2 peaks appeared in the spectra with lower intensities at 18, 34.2, and 51.8°, as result of CaO hydration caused by moisture from the environment at ambient temperature. Furthermore, the peak corresponding to Ni was detected at 44.6°, indicating the reduction of NiO to metallic Ni after catalyst reduction. However, the presence of Ni is hard to rule out at 5 wt% loading in the XRD patterns, owing to better dispersion of Ni0 on the CaO surface, even with increasing ZnO addition (Fig. 2a–c). Similar observation was reported by Xia et al. (2012), where no obvious diffraction peak observed with 3 and 9 wt% of Ni loading on SiO2 due to smaller NiO particles size which is highly dispersed on the support [18]. However, the peak intensity relative to Ni was found to increase with Ni loading, owing to possible growth in the Ni crystallites caused by weak interactions with the support. Apart from this, another obvious diffraction peak at 43.1° is possibly caused by the formation of ZnxNi1−x O phases, which could be caused by the strong interactions between NiO and ZnO on the support. Besides, the ability of larger Zn2+ to substitute Ni2+ in the NiO lattice facilitates solid solution formation, caused by the ability of Zn to dissolve into the NiO rocksalt lattice [19]. Table 1 summarizes the average CaO and Ni cluster size of the catalysts, which was calculated from the diffraction spectra and Scherrer equation. The CaO crystallite sizes vary slightly in the range of 41–57 nm, owing to the incorporation of various added dopants, which tend to create defects and alter the crystallographic structure. At a lower concentration (5 wt%), the particle sizes of Ni are difficult to calculate, caused by high dispersion on the support, as mentioned earlier based on the XRD peaks. However, the presence of metallic Ni peaks with higher loadings is caused by particle agglomeration, where the sizes are in the range of 30–40 nm. The BET surface areas of the catalysts and the pore volumes were measured using N2 adsorption/desorption techniques and the results are presented in Table 1. Bulk CaO catalyst exhibited a small surface area (~ 5.5 m2 g−1) and low porosity [20], and similar properties were observed with all of the prepared catalysts with the addition of ZnO and Ni on CaO. The characterized catalysts show low surface areas in the range of 2.8–4.9 m2 g−1, which were affected by the added dopants.
Temperature-Programmed Desorption–CO2 Analysis
The basicity of the catalysts and the basic site distribution were measured using TPD–CO2 analysis. The total amounts of CO2 desorption and the absorption strength as functions of temperature are presented in Table 2 and the TPD–CO2 profile can be seen in Fig. 3. Table 2 shows that all of the prepared catalysts show a basic nature as a result of CaO intervention as the support, which dominates the overall catalyst properties. However, the addition of Zn and Ni altered the basicity and the distribution of sites, where a slight variance is noted with the amount of added dopants. The basicity of the catalysts with increased Zn loading was found to increase up to 10 wt% and a minor drop occurred at a loading of 15 wt%. Ni loading in the range of 5–15 wt% into CaO that contained the same amount of ZnO shows a clear increasing trend in the catalyst basicity. However, Ni and ZnO were reported to have no significant contribution in terms of basic site increments, whereby both were considered to not have notable basic properties [21, 22]. Thus, the distortion and defects that have been created by strong interactions between the added dopants on the CaO crystals structure were responsible for changing the basicity of the prepared catalysts. Basicity was found to be increased with the incorporation of NiO into the CaO lattice due to the existence of strong basic sites that formed between the binary oxides [23].
Temperature-Programmed Reduction–H2 Analysis
It was reported that the reduction of NiO is necessary when the presence of a Ni phase on the support was found to be active in catalyzing the SCWG reaction and elevating hydrogen production [11, 24]. Thus, all of the prepared catalysts were reduced in 5% H2/Ar flow at 700 °C for 3 h. Thus, TPR analyses were carried to study the reducibility of the catalysts, which will provide a basic understanding of the dopants interaction on the CaO surface. Reducibility of the individual components of the catalysts, including CaO, NiO, and ZnO that have also been reported previously, whereby bulk CaO and ZnO are hard to reduce, as both oxides possess very strong Ca–O and Zn–O interactions [25]. However, from our TPR analyses, we found a very small reduction peak for bulk CaO at 610.3 °C, which was probably caused by a weak Ca–O bond reduction at the surface (not shown here). In addition, bulk NiO tends to be reduced at temperatures in the range of 400–418 °C, where flowing hydrogen scavenges the lattice oxygen (NiO + H2 ➔ Ni + H2O) and leaves active metallic Ni on the catalyst [26]. However, the difference in the reduction temperature in the formulated catalysts compared with the bulk condition can postulate the strength of the dopant attraction on the CaO surface, which can be classified into weak (200–400 °C), medium (400–600 °C), and strong interactions (> 600 °C) based on the reduction peak occurrence relative to the temperature. The TPR profiles show that there are two distinctive peaks in the temperature range of 450–600 °C and above 700–900 °C (Fig. 4). This is attributed to the strength of the interaction of NiO on the CaO surface, as both CaO and ZnO are difficult to reduce. Addition of nickel into the CaO catalyst shows a reduction peak at 555.4 °C, which appears close to the first temperature range. Thus, it can be concluded that the first reduction peak is caused by medium interactions between NiO and the CaO surface. However, with the addition of a secondary dopant (ZnO), a second peak appears in a higher temperature range, indicating very strong interactions between NiO and the catalysts. This is attributed to several possibilities such as new interaction establishment between NiO and the ZnO dopant or the formation of smaller NiO particles dispersed on the CaO surface, which shift the reduction temperature to the right. According to previously published reports, bulk and larger NiO particles tend to be reduced at lower temperatures compared with the smaller-sized particles that form very strong interactions with the support [27]. However, from the XRD patterns of the synthesized catalysts, the presence of a Ni.8Zn.2O phase was identified in all of the prepared catalysts as result of strong interactions between NiO and ZnO on the support. Incorporation of the added dopants shift the reduction peak to higher temperatures, where some of the NiO remains unreduced at 700 °C under a partial hydrogen flow. Furthermore, Table 2 summarizes the reducibility of each prepared catalyst and its reduction peak relative to temperature. It was found that only slight changes in H2 consumption were observed with increasing ZnO, owing to its non-reducible behavior and the similar NiO content on the catalysts. Besides, the appearance of a Ni.8Zn.2O peak is more visible when the reduction peaks are observed at 893, 900, and 863 °C with increasing ZnO loading. Furthermore, a higher amount of H2 consumption was noticed in the reduction of the catalysts (Ni2+ ➔ Ni0) with increasing Ni loading. However, the TPR profile (Fig. 4) shows that increasing the Ni loading causes a new peak to form at 650 °C, which overlaps with other reduction peaks that existed previously. Thus, swelling in the region at 600–750 °C and shifting of the second reduction peak to a lower temperature indicates the possibilities of Ni particle agglomeration at higher concentrations on the CaO. In addition, increasing the Ni loading accelerates the nucleation of larger NiO crystallites and causes the high-temperature reduction peak to shift to lower temperatures, owing to its weak interaction with the support [28].
Catalytic Supercritical Water Gasification
SCWG reaction is a promising technique for biomass conversion into hydrogen-rich product gas to overcome biomass with high water content. There have been many plausible reaction mechanisms proposed in previous reports that represent the complex pathway of the reaction. However, the overall chemical conversion can be simplified as follows (Eq. (1)) [29]:
where x and y represent the elemental molar composition of H/C and O/C in biomass.
Based on the defined reaction mechanism, the overall product gas compositions from the SCWG reaction can be predicted to mainly consist of H2 and CO2. However, a small amount of the gas includes CO, and hydrocarbons (C1–C4) also tend to be produced during the reaction. Besides the summarized reaction mechanism, in reality, there are many intermediate reactions, which include steam reforming (Eq. (2)), methanation (Eq. (3)), and WGS reactions (Eq. (4)).
In addition, the reaction temperature and catalyst used in the SCWG reaction significantly influence the reaction efficiency and product gas composition. The reaction temperature has been classified into three main categories: < 374 °C (subcritical), 374–500 °C, and > 500 °C [29]. However, water reaches a supercritical state at ≥ 374 °C, where its specific properties enhance biomass gasification to produce hydrogen-rich product gas. Catalyst are applied in order to enhance selectivity towards hydrogen and to enable biomass conversion at lower reaction temperatures. There are various types of catalysts that have been reported, such as metallic Ni-based catalyst, activated carbon, noble metals, and several oxides including ZrO2, CaO, and CeO2 [12, 14, 17, 24, 29,30,31]. Based on our preliminary studies, we found that ZnO-doped Ni–CaO catalysts significantly enhance hydrogen production during EFB SCWG at 380 °C [32]. The promotional effects of ZnO dopant on SCWG catalyst such as strong basicity and water splitting capabilities were found to be a major factor in the increment of hydrogen yield [33]. Furthermore, the synergic effect of the combination of CaO, ZnO, and Ni leads to greater promotional effects in the WGS reaction, showing that a major source of hydrogen production in the SCWG reaction. Apart from its use as the reactant medium, the tendency of water participation in the WGS reaction represents a promising way to elevate the overall hydrogen production. Thus, the preparation of ZnO-doped Ni–CaO with different dopant loadings is essential in order to optimize the catalyst formulation to achieve higher catalytic activities.
Effect of ZnO Loading on 5 wt% Ni–CaO
Figure 5 shows the product gas composition caused by ZnO dopant loading (5, 10, and 15 wt%) onto 5 wt% Ni–CaO catalysts during EFB SCWG at 380 °C. The gas compositions were slightly varied with catalysts, as a result of the different amount of ZnO present in the catalyst. Product gas composition shows that the major part of the gas consisted of H2 and CO2, whereas CO and CH4 were measured in very low concentrations. Similar to the postulated biomass-conversion pathway, according to Eq. (1), the gases were produced from various reaction mechanisms and structural cleavages, which took place under supercritical conditions. Catalytic SCWG of EFB with increasing ZnO loading (5, 10, and 15 wt%) on Ni–CaO catalysts does not improve the hydrogen production and started to drop from 105.7 (5 wt%) to 94.1 (10 wt%) and 76.8 mmol mL−1 (15 wt% loading). The main sources of hydrogen produced during SCWG are from the WGS reaction and from hydrocarbon-reforming reactions. The presence of ZnO in Ni–CaO catalyzes the WGS reaction, whereby increasing the loading beyond 5 wt% reduces the hydrogen concentration. Increasing ZnO addition shows a negative effect on the WGS reaction. This phenomenon is possibly caused by ZnO agglomeration, owing to large loading and coverage of the active sites of CaO, which are involved in the gasification reaction. Besides, the present of Ni.8Zn.2O phase on CaO initiates synergistic effects, which ease the WGS reaction. Thus, the stability of the Ni.8Zn.2O phase is vital to promote WGS under harsh supercritical conditions. The prediction correlates with the performance of the catalysts, where the presence of a Ni.8Zn.2O peak in TPR shifts to a lower temperature with increasing ZnO loading. Besides, several previously published reports have mentioned that the presence of metallic Ni also promotes the WGS reaction along with increased carbon conversion [14, 34, 35]. However, the methanation reaction, classified as the hydrogenation of CO and CO2 by the metallic Ni dopant, has notable drawbacks, which tend to reduce hydrogen production. Apart from this, CaO catalysts have shown significant contributions to high hydrogen production, due to its dual role as a CO2 sorbent and as a catalyst during the EFB SCWG reaction. The potential application of CaO in SCWG was reported by Zhang et al. [17], using coal–water slurry SCWG reaction, where CaO was found to catalyze the gasification, WGS, and reforming reactions. Furthermore, the basic nature of CaO offers an additional advantage to promote the WGS, which is propelled by the cyclic coordination of intermediate anions that are involved in hydrogen production. It has been described based on carbonate conversion to formate in the presence of CO and water that form an intermediate compound (formaldehyde) which decomposes to H2, CO, and CO32−. Subsequently, CO and CO32− ions are used back in the reaction mechanism where CO2 released at the end [36].
Effect of Reaction Time on ZnO-Doped 5 wt% Ni–CaO
From our experiments in the absence of a catalyst, the reaction time of EFB SCWG was found to have no effect on the product gas composition or feedstock gasification. This is attributed to the formation of thermally stable tarry compounds, which are hard to degrade at the reaction temperature. Thus, increasing reaction time shows less impact on the overall product gas composition and only a slight variation is observed in the amount of H2 and CO, caused by the progressing WGS reaction with time. Thus, the effects of reaction time on the overall product gas composition (CO2, H2, CH4, CO) in the presence of the prepared catalysts are presented in Fig. 6. The results show that notable differences in the product gas composition occurred with increasing reaction time for the added catalysts reaction. However, there are no clear patterns in CO2 production with increasing reaction time using the added catalysts. Plausible ways to CO2 production are mainly through carboxyl group cleavages from the biomass structures [37] or CO conversion through the WGS reaction. An almost similar CO2 concentration shows that there is no further degradation in the dissolved biomass with increasing time or with increasing ZnO loading on the added catalysts. However, H2 production is directly proportional to reaction time, whereby its concentration linearly increases with time. Even though the addition of catalysts altered the level of hydrogen production, a steadily increasing trend is seen with increasing reaction time. This phenomenon postulates that the progressing WGS and reforming reactions, aided by the added catalysts with increasing reaction time, elevate the overall level of hydrogen production. Among the catalysts, the 5% ZnO-doped catalyst shows steady H2 production with increasing reaction time, in contrast to the 10 and 15% doped alternatives. This shows that increasing the ZnO content has negative effects in terms of hydrogen-favored reactions. In conclusion, the 5% ZnO-doped catalyst was found to be promising, whereby a steady increase in hydrogen production was observed with increasing reaction time from 8 to 32 min due to progressing WGS reaction. In addition, the concentration of CH4 shows a linear increase with reaction time. Generally, CH4 production was linked to two factors, including functional group cleavage and methanation reaction [14, 38]. It is reported that CH4 mainly produced from breaking of functional groups (O–CH3) [34], where the dissolved liquid oxygenates from EFB slowly degraded in the supercritical environment [38]. Besides, numerous reports have stated that the methanation reaction is highly favored in the presence of metallic Ni catalysts [31, 39]. Even though CH4 production is relatively low compared with H2 and CO2, its increment could still cause a reduction in the H2 yield. Among the catalysts, 5% ZnO-doped catalyst shows a higher tendency for CH4 production, which is possibly caused by well-dispersed metallic Ni on the catalyst surface without strong interactions with ZnO. Besides, the CO concentration shows a steady reduction with increasing reaction time, as a result of the advancing WGS reaction caused by the added catalysts, as predicted earlier. Thus, from the CO reduction patterns over time, the 5 wt% ZnO-doped catalyst caused a drastic drop at 16-min reaction time compared with other catalysts, whereas a further increase in time shows a similar concentration. It can be concluded that, among the tested catalysts, with increasing reaction time (8–32 min), the 5 wt% ZnO-doped Ni–CaO catalyst exhibits high catalytic activity towards hydrogen production. However, in terms of overall carbon concentration in the gas, the phases show similar patterns (15–18 mmol mL−1) and residues are found in the reactor even after prolonging the reaction time (Table 3). This shows that complete carbon conversion is hard to achieve, even with catalyst addition, owing to lower reaction temperature (380 °C). However, the application of Ru catalyst in SCW reaction at the similar condition to the current investigation shows catalytic activity maintained with an increase in reaction time (5–15 min). In addition, carbon yield linearly improved [40] compared with 5 wt% ZnO-doped Ni–CaO catalyst where carbon gasification was stagnant with an increase in reaction time. This is possibly due to the Ni particles sintering or potential surface oxidation of Ni which lowers the catalytic activity and gas yield.
Besides, reaction temperatures in the range of 380–600 °C were considered lower temperatures for SCWG, whereby complete carbon conversions are difficult to achieve even with the addition of catalysts [4]. Generally, noble metal catalysts (Ru) are employed, which give considerable carbon conversion at lower reaction temperatures [4, 41]. However, noble metals are associated with higher catalyst costs whereby Ni-based catalysts are preferred and selected for SCWG. Despite several drawbacks such as catalyst deactivation, methanation reaction, and higher reaction temperature requirement (> 450 °C), the catalyst performance can be enhanced with the addition of dopants. Besides, the nature of EFBs also contributes to the lower carbon conversion, which is caused by its complex intrinsic components. Differences in the thermal stability of each component in EFBs dictate the overall degradation patterns and greatly influence the product gas composition and residue formation. In comparison with EFBs, which consist of a complex structure (lignin, cellulose, and hemicellulose), model compounds are generally used to study degradation patterns in SCWG. Among the components, lignin tends to produce the highest number of residues and it is hard to gasify, aided by its strong physical structure compared with other lignocellulosic model compounds like cellulose, hemicellulose, and glucose [34]. Thus, the combination of these compounds in EFBs also influences its degradation compared with pure lignocellulosic model compounds.
Effect of Ni Loading on 5 wt% ZnO–CaO Catalysts
Apart from ZnO loading, the effect of Ni addition (5–15 wt%) on CaO in EFB SCWG is reported in Fig. 7. The product gas composition varies slightly with increasing Ni addition to the catalysts. It was found that increasing the Ni loading (5, 8, 10, 12, 15 wt%) does not show any significant improvements in H2 production, where a reduction in the hydrogen concentration is observed. Thus, it can be concluded that there is no further enhancement in the WGS and reforming reactions with increased Ni loading. Generally, increasing the metal loading could lead to particle agglomeration by a sintering effect on the catalysts surface, thereby reducing catalytic activity. Growth of the Ni particles under SCWG conditions causes a reduction in the number of active sites and leads to intense catalyst deactivation [42]. This phenomenon can be seen in the poor catalyst performances in terms of similar hydrogen yield and carbon gasification, even with increased Ni loading. Besides, the presence of overlapping peaks at 700 °C in the TPR profile with Ni addition indicates possible particle agglomeration on the catalysts. Generally, highly dispersed, smaller-sized Ni particles tend to form very strong bonds with the support and exhibit a high-temperature reduction peak [27]. However, the formation of larger Ni particles could cause a shift in the reduction temperature to the left, in agreement with weak interactions between support added Ni [28]. In addition, such transitions on the catalyst reduction peaks observed in the TPR profiles are caused by the presence of larger Ni particles, which show lower catalytic activity with increasing Ni loading. Furthermore, the production of CO2, CO, and CH4 is highly influenced by the presence of metallic catalysts, as they are mainly produced from the degradation of dissolved EFB compounds. Metallic Ni was reported to be very active in C–C bond breaking and in promoting gasification reactions. Table 3 shows the carbon concentration in the gas phase, which shows the ability of the catalysts to promote carbon conversion. The carbon concentration increased slightly after 5–8 wt% Ni addition, but then dropped with further increase, due to possible particle agglomeration phenomena, as described above. Furthermore, the 8% Ni-doped catalyst shows the highest production of CO2, CO, and CH4, which are mainly produced from functional group cleavages and rapturing dissolved liquid oxygenates from EFBs. Among the gases produced, the amount of CO shows a significant difference with the added catalysts, whereby the production increased with up to 8 wt% Ni loading and dropped with further increase. However, based on the H2 concentration obtained, it can be concluded that changes in the amount of CO were not fully influenced by the WGS reaction, where there was no increase in the amount of H2 produced with CO reduction. Thus, a lower tendency for functional group cleavages and reduced C–C bond rupture are favored at higher Ni loadings. A loading of 5–8 wt% Ni was found to be adequate with 5 wt% ZnO loading on CaO catalysts, which improved hydrogen production through WGS and reforming reactions.
Conclusion
This investigation shows the optimum formulation of CaO-based catalysts with the addition of ZnO and Ni. Increasing the ZnO loading from 5 to 15 wt% does not show a notable improvement in terms of hydrogen production. In fact, 10 and 15 wt% ZnO-doped catalysts led to a decrease in hydrogen production. In addition, a Ni loading above 8 wt% reduced the catalytic activity because of particle agglomeration. Thus, a loading of 5–8% Ni was found to be appropriate to catalyze C–C bond breaking, as indicated by the higher carbon concentration in the product gas. Thus, among the prepared catalysts, 5 wt% ZnO with a Ni loading of 5 wt% on CaO was found to be the best catalyst, based on the highest hydrogen production and feedstock gasification. The synergetic effects of the Ni.8Zn.2O phase in the CaO catalyst significantly promote the water gas shift reaction and elevate the overall gas production. Besides, reaction time shows no further improvement in term of carbon conversion as a result of thermally stable tarry compounds formation. However, the gas composition changed, due to various reaction mechanisms progressing simultaneously, including the WGS and methanation reactions. Hydrogen projected an increasing trend over time, boost by a progressing WGS reaction, as indicated by the decreasing amount of CO. In addition, the CH4 concentration was found to increase over time, which was caused by both functional group cleavage and the methanation reaction aided by the presence of metallic Ni catalysts. Thus, the addition of ZnO and Ni to an appropriate amount of CaO produced an effective catalyst that shows high selectivity for hydrogen production through an enhanced WGS reaction.
References
Rodriguez Correa C, Kruse A (2018) Supercritical water gasification of biomass for hydrogen production-review. J Supercrit Fluids 133:573–590
Phang KY, Lau SW (2017) A survey on the usage of biomass wastes from palm oil mills on sustainable development of oil palm plantations in Sarawak. IOP Conf Ser: Mater Sci Eng 206:012091. https://doi.org/10.1088/1757-899X/206/1/012091)
Agensi Innovasi Malaysia (2013) National Biomass Strategy 2020: new wealth creation for Malaysia’s biomass industry; AIM: Selangor, Malaysia
Matsumura Y, Minowa T, Potic B, Kersten SRA, Prins W, van Swaaij WPM, de Beld BV, Elliott DC, Neuenschwander GG, Kruse A, Antal MJ Jr (2005) Biomass gasification in near- and super-critical water: status and prospects. Biomass Bioenergy 29:269–292
Watanabe M, Inomata H, Osada M, Sato T, Adschiri T, Arai K (2003) Catalytic effects of NaOH and ZrO2 for partial oxidative gasification of n-hexadecane and lignin in supercritical water. Fuel 82(5):545–552
Wang J, Takarada T (2001) Role of calcium hydroxide in supercritical water gasification of low-rank coal. Energy Fuel 15(2):356–362
Schmieder H, Abeln J, Boukis N, Dinjus E, Kruse A, Kluth M, Petrich G, Sadri E, Schacht M (2000) Hydrothermal gasification of biomass and organic wastes. J Supercrit Fluids 17(2):145–153
Kruse A, Faquir M (2007) Hydrothermal biomass gasification – effects of salts, backmixing, and their interaction. Chem Eng Technol 30(6):749–754
Xu D, Wang S, Hu X, Chen C, Zhang Q, Gong Y (2009) Catalytic gasification of glycine and glycerol in supercritical water. Int J Hydrogen Energ 34:5357–5364
Guo Y, Wang SZ, Xu DH, Gong YH, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew Sust Energ Rev 14(1):334–343
Azadi P, Farhood R (2011) Review of heterogeneous catalyst for sub- and supercritical water gasification of biomass and wastes. Int J Hydrogen Energ 36:9529–9541
Xu X, Matsumura Y, Stenberg J, Antal MJJ (1996) Carbon-catalyzed gasification of organic feedstocks in supercritical water. Ind Eng Chem Res 35(8):2522–2530
Yamaguchi A, Hiyoshi N, Sato O, Bando KK, Osada M, Shirai M (2009) Hydrogen production from woody biomass over supported metal catalysts in supercritical water. Catal Today 146(6):192–195
Azadi P, Afif E, Azadi F, Farnood R (2012) Screening of nickel catalyst for selective hydrogen production using supercritical water gasification of glucose. Green Chem 14:1766–1777
Osada M, Sato O, Arai K, Shirai M (2006) Stability of supported ruthenium catalysts for lignin gasification in supercritical water. Energy Fuel 20(6):2337–2343
Cao C, Zhang Y, Cao W, Jin H, Guo L, Huo Z (2017) Transition metal oxides as catalysts for hydrogen production from supercritical water gasification of glucose. Catal Lett 147:828–836
Zhang R, Jiang W, Cheng L, Sun B, Sun D, Bi J (2010) Hydrogen production from lignite via supercritical water in flow-type reactor. Int J Hydrogen Energ 35(21):11810–11815
Xia WX, Hou YH, Chang G, Weng WZ, Han GB, Wan HL (2012) Partial oxidation of methane into syngas (H2 + CO) over effective high-dispersed Ni/SiO2 catalysts synthesized by a sol–gel method. Int J Hydrogen Energ 37:8343–8353
Gaskell KJ, Starace A, Langell MA (2007) ZnxNi1-xO rocksalt oxide surfaces: novel environment for Zn2+ and its effect on the NiO band structure. J Phys Chem C 111:13912–13921
Taufiq-Yap YH, Sivasangar S, Salmiaton A (2012) Enhancement of hydrogen production by secondary metal oxide dopants on NiO/CaO material for catalytic gasification of empty palm fruit bunches. Energ 47:158–165
Garcia V, Fernandez JJ, Ruiz W, Mondragon F, Moreno A (2009) Effect of MgO addition on the basicity of Ni/ZrO2 and on its catalytic activity in carbon dioxide reforming of methane. Catal Commun 11(4):240–246
Taufiq-Yap YH, Lee HV, Hussein MZ, Yunus RR (2011) Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcasoil to biodiesel. Biomass Bioenergy 35:827–834
Teo SH, Rashid U, Taufiq-Yap YH (2014) Biodiesel production from crude Jatropha Curcas oil using calcium based mixed oxide catalysts. Fuel 136:244–252
Yan B, Wu J, Xie C, He F, Wei C (2009) Supercritical water gasification with Ni/ZrO2 catalyst for hydrogen production from model wastewater of polyethylene glycol. J Supercrit Fluids 50(2):155–161
Tang CW, Chuang SSC (2014) The effect of reduction of pretreated NiO-ZnO catalysts on the water gas shift reaction for hydrogen production as studied by in situ DRIFTS/MS. Int J Hydrogen Energ 39(2):788–797
Brown R, Cooper ME, Whan DA (1982) Temperature programmed reduction of alumina-supported iron, cobalt and nickel bimetallic catalysts. Appl Catal 3:177–186
Zhang X, Liu J, Jing Y, Xie Y (2003) Support effects on the catalytic behavior of NiO/Al2O3 for oxidative dehydrogenation of ethane to ethylene. Appl Catal A: Gene 240(1–2):143–150
Li C, Chen YW (1995) Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: effects of the preparation method. Thermochim Acta 256(2):457–465
Barati M, Babatabar M, Tavasoli A, Dalai AK, Das U (2014) Hydrogen production via supercritical water gasification of bagasse using unpromoted and zinc promoted Ru/γ-Al2O3 nano catalysts. Fuel Process Technol 123:140–148
Sato T, Inda K, Itoh N (2011) Gasification of bean curd refuse with carbon supported noble metal catalysts in supercritical water. Biomass Bioenergy 35(3):1245–1251
Hao X, Guo L, Zhang X, Guan Y (2005) Hydrogen production from catalytic gasification of cellulose in supercritical water. Chem Eng J 110(1–3):57–65
Sivasangar S, Mastuli MS, Islam A, Taufiq Yap YH (2015) Screening of modified CaO-based catalysts with a series of dopants for the supercritical water gasification of empty palm fruit bunches to produce hydrogen. RSC Adv 5:36798–36808
Mastuli MS, Kamarulzaman N, Kasim MF, Sivasangar S, Saiman MI, Taufiq-Yap YH (2017) Catalytic gasification of oil palm frond biomass in supercritical water using MgO supported Ni, Cu and Zn oxides as catalysts for hydrogen production. Int J Hydrog Energy 42:11215–11228
Azadi P, Khan S, Strobel F, Azadi F, Farnood R (2012) Hydrogen production from cellulose, lignin, bark and model carbohydrates in supercritical water using nickel and ruthenium catalysts. Appl Catal B Environ 117-118:330–338
Lee IG (2011) Effect of metal addition to Ni/activated charcoal catalyst on gasification of glucose in supercritical water. Int J Hydrogen Energ 36(15):8869–8877
Elliott DC, Hallen RT, Sealock LJ Jr (1983) Aqueous catalyst systems for the water-gas shift reaction. 2. Mechanism of basic catalysis. Ind Eng Chem Prod Res Dev 22:431–435
Xu CC, Donald J (2012) Upgrading peat to gas and liquid fuels in supercritical water with catalysts. Fuel 102:16–25
Sivasangar S, Zainal Z, Salmiaton A, Taufiq-Yap YH (2015) Supercritical water gasification of empty fruit bunches from oil palm for hydrogen production. Fuel 143:563–569
Minowa T, Zhen F, Ogi T (1998) Cellulose decomposition in hot-compressed water with alkali or nickel catalyst. J Supercrit Fluids 13(1–3):253–259
Tiong L, Komiyama M (2019) Supercritical water gasification of microalga Chlorella vulgaris over supported Ru. J Supercrit Fluids 144:1–7
Osada M, Sato T, Watanabe W, Adschiri T, Arai K (2004) Low-temperature catalytic gasification of lignin and cellulose with a ruthenium catalyst in supercritical water. Energy Fuel 18:327–333
Lu Y, Zhu Y, Li S, Zhang X, Guo L (2014) Behavior of nickel catalysts in supercritical water gasification of glucose: influence of support. Biomass Bioenergy 67:125–136
Funding
The authors express great appreciation to financial support from the Ministry of Higher Education of Long Term Research Grant Scheme (LRSG) NanoMITE (vot no. 5526308) and Universiti Putra Malaysia.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taufiq-Yap, Y.H., Sivasangar, S. & Surahim, M. Catalytic Supercritical Water Gasification of Empty Palm Fruit Bunches Using ZnO-Doped Ni–CaO Catalyst for Hydrogen Production. Bioenerg. Res. 12, 1066–1076 (2019). https://doi.org/10.1007/s12155-019-10031-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10031-8