Abstract
The lipase-immobilized polyethyleneimine (PEI)- and polyacrylic acid (PAA)–coated magnetic silica nanocomposite particles (L-PEI-MS and L-PAA-MS, respectively) were prepared and applied at various transesterification reaction conditions. The reactions were carried out with soybean, sunflower, canola, and palm oils along with methanol or ethanol in the solvent-free and n-hexane systems. The highest fatty acid methyl ester (FAME) and fatty acid ethyl ester (FAEE) synthesis yields were obtained from the transesterification of palm oil, i.e., almost 7.7–10.2% higher than other oils. At the constant reaction conditions, the application of ethanol leads to higher (6.0–8.7%) reaction yields in comparison with methanol. In addition, irrespective of reaction conditions, the best performance was acquired by L-PEI-MS; e.g., the FAME and FAEE synthesis yield values of 81.2% and 88.3% were obtained from transesterification of palm oil in the solvent-free systems, respectively. Addition of n-hexane improved the synthesis of FAME and FAEE yield values to 88.9% and 93.3%, respectively, using L-PEI-MS. The transesterification reactions kinetics follows the Ping-Pong Bi-Bi mechanism with alcohol inhibition effects. The high catalytic performance of L-PEI-MS might be related to its hydrophobic nature, which enhances the accessibility of oil molecules to the immobilized lipases and hampers the deactivating effect of alcohol molecules on them.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The enzymatic transesterification of oils using immobilized enzymes for biodiesel production, defined as fatty acid alkyl esters, has recently become an attractive approach in comparison with chemical catalysis methods, because it offers recoverable biocatalysts, green and mild reaction conditions, elimination of saponification, high-purity products, and facile product separation [1,2,3]. Biodiesel is known as a renewable, biodegradable, and non-toxic fuel [4, 5]. Although there is a controversy regarding the application of edible vegetable oils for biodiesel production, considering their relatively high price, they are still the main sources used in the biodiesel industry. This is mainly due to the fact that alternative low-cost feedstock such as waste oils and animal fats contain a large amount of free fatty acids, which reduce biodiesel synthesis efficiency, and downstream processing is needed for the mass production of biodiesel from such oils [5, 6]. Another low-cost feedstock can be microalgae, but commercial microalgal biodiesel production is not yet economically viable, largely because of their low lipid content [7]. Therefore, the most important vegetable oils produced worldwide for the purpose of biodiesel production are palm, soybean, sunflower, and canola oils. Generally, sunflower and canola oils are most commonly used in Europe, soybean oil predominates in the USA, and palm oil is principally used in tropical countries [6,7,8].
The immiscibility of substrates, i.e., alcohol and oil, and the enzyme deactivation by alcohol through stripping water off of the enzymes’ surface have however remained two critical issues in enzymatic transesterification reactions [5, 9]. These types of problems can be minimized by employing an organic solvent in the reaction systems. According to the wide range of studies [10,11,12], organic solvents may improve the solubility of alcohol and oil and limit the alcohol concentration surrounding enzymes, which in turn result in high stability of enzymes and high fatty acid alkyl esters synthesis yield. For example, it has previously been reported that the choice of solvent can have a significant effect on the reaction yield values ranging from 2.7 to 87%, in the transesterification of soybean oil. It was also shown that solvents from the alkane group result in the highest biodiesel yields, particularly n-hexane and isooctane [10].
The implementation of other types of alcohols instead of methanol, which is most frequently used in biodiesel production because of cost reasons, can also lessen the deactivation of enzymes [13]. For example, ethanol being a cheap and abundant commodity produced from the fermentation of sucrose from sugarcane promotes the miscibility between the alcohol and the oil phases [14,15,16]. Naranjo et al. [13] reported higher biodiesel synthesis yield value of 57% through the transesterification of palm oil with ethanol using lipase from Candida antarctica B immobilized on activated carbon, compared with that obtained using methanol (48%).
The problems contributed to the enzymatic transesterification reactions can be also addressed by improving the structural and physicochemical characteristics of the supports used for the enzyme immobilization. Porous structures like silica and polymer resins have been reported as suitable support materials for enzyme immobilization in order to reduce direct interactions of enzymes and alcohol molecules [17,18,19]. For instance, Kalantari et al. [18] reported core-shell-structured magnetic mesoporous silica nanoparticles as a suitable recoverable support material for the immobilization of lipase and subsequently production of biodiesel from soybean oil with the relatively high yield value of 55% in comparison with that of free lipase (49%). In another study, it has been shown that the lipase entrapped in silica sol-gel can perform more effectively for the production of 67% biodiesel from soybean oil, as compared with the free lipase giving a yield of 40% [19]. It has also been reported that the lipases physically adsorbed onto NKA (No1KCla-activated carbon) macroporous resin can result in a 98% biodiesel production yield value [17]. However, the complicated process of the synthesis of porous materials and high mass transfer resistance in the pores limit the application of such materials. The hydrophobic/hydrophilic characteristics of immobilization supports can be an important aspect which has been considered only in a few studies including our recent studies [20,21,22]. Ferrario et al. [21], through studying the FAME synthesis from coffee waste oil, observed higher catalytic activity of the lipase from Candida antarctica immobilized onto styrenic porous resin (being a hydrophobic support, giving a yield of 54%), as compared with that of the lipase immobilized on methacrylic porous resin (being a hydrophilic support giving a yield of 29%).
To address the above-mentioned challenges, we aimed to develop two different types of polymer-coated Fe3O4 cluster@ SiO2 (MS) particles and investigate their physicochemical properties, as well as reaction conditions, in order to achieve high efficiency of transesterification of oils. In our previous report [22], we investigated and optimized some of the important parameters influencing the efficiency of soybean oil transesterification reaction such as the type and molecular weight of polymers (PEI and PAA) coated on the magnetic particles, reaction time, methanol:oil molar ratio, steps of methanol addition, water:oil mass ratio, and reaction temperature. It was also represented that the PEI-MS particles with superior hydrophobic properties are able to absorb higher values of the oil molecules that limit the absorption of alcohol molecules and consequently preserve immobilized enzymes from deactivation by alcohol, which all resulted in higher reaction efficiencies in comparison with PAA-MS particles with superior hydrophilic properties [22]. In this research, the transesterification reactions are carried out using various types of plant oils commonly used in the production of biodiesel, including palm, soybean, sunflower, and canola oils, to evaluate the effect of oil source on the reaction yields. For the sake of comparison, two types of alcohols, i.e., methanol and ethanol, are used in the reaction media. The reactions are conducted in both solvent-free and n-hexane systems. The effect of alcohol to oil molar ratio is also undertaken. Eventually, the reaction kinetics and the stability of the prepared magnetically recoverable biocatalysts are studied.
Materials and Methods
Chemicals
Ethanol, methanol, n-hexane, and glutaraldehyde solution (GA, 25%) were purchased from Merck. Polyethyleneimine (PEI, average molecular weight 750,000), polyacrylic acid (PAA, average molecular weight 100,000), N-hydroxy succinimide (NHS), N-(3-dimethylaminopropyl)-N ethyl carbodiimide hydrochloride (EDC), methyl heptadecanoate (MH, a chromatographic standard), and lipase from Pseudomonas cepacia (EC 3.1.1.3) were received from Sigma-Aldrich. All reagents were used as received without further purification. The refined soybean, sunflower, canola, and palm oils were purchased from Jahan Plant Oil Company (Tehran, Iran). Milli-Q water (18.2 MΩ cm) was used throughout the work. The aqueous sodium phosphate buffer solution (0.1 M and pH 7.4) was used throughout the work.
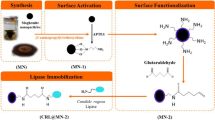
Synthesis of Lipase-Immobilized PEI- and PAA-Coated Magnetic Silica Nanocomposite Particles
To prepare the lipase-immobilized polymer-coated MS particles, first, the [3-(2-aminoethyl amino) propyl] trimethoxy silane-functionalized MS particles (AAS-MS) were synthesized as described previously [22,23,24]. In order to prepare PEI-MS particles, briefly, 100 μL of GA was mixed with 5 mg of AAS-MS particles dispersed in 2.5 mL sodium phosphate buffer solution (0.1 M and pH 7.4) at 4 °C for 1 h. After washing and dispersion of the activated AAS-MS particles in 2.5 mL of the buffer solution, 2.5 mL of 2.7 × 10−3 mM solution of PEI was added into the particle suspension and the mixture was shaken for 2 h. The synthesized PEI-MS sample was finally washed and preserved in 2.5 mL of the buffer solution [23].
To synthesize the PAA-MS particles, 2.5 mL of the mixed solution of EDC (10.43 mM) and NHS (3.48 mM) in the buffer solution was firstly added to 1.25 mL of 0.02 mM solution of PAA and the mixture was shaken for 10 min. The activated PAA solution was then added to the suspension of 5 mg of AAS-MS particles in 1.25 mL of the buffer solution. Finally, after 2-h reaction, the synthesized PAA-MS sample was washed and kept in 2.5 mL of the buffer solution [24].
In order to immobilize the lipase onto the PEI-MS particles, 100 μL of GA was added to the prepared PEI-MS particles solution and incubated at 4 °C for 1 h. After washing and dispersion of particles in 2.5 mL of the buffer solution, the lipase solution (5 mg in 2.5 mL of the buffer solution) was added to the particle suspension. After overnight incubation, the lipase-immobilized PEI-MS (L-PEI-MS) sample was washed and stored in the buffer solution at 4 °C [23].
To immobilize the lipase onto the PAA-MS particles, 2.5 mL of the mixed solution of EDC (10.43 mM) and NHS (3.48 mM) in the buffer solution was added to the solution of the prepared PAA-MS particles and shaken for 10 min. Once the particles were separated and re-dispersed in 2.5 mL of the buffer solution, 2.5 mL of the lipase solution (2 mg mL−1) was quickly added to the particles suspension and the mixture was shaken at 4 °C for 2 h. Finally, the lipase-immobilized PAA-MS (L-PAA-MS) sample was washed and maintained in the buffer solution at 4 °C [24].
Enzymatic Transesterification of Oils
In all of the transesterification reactions, 0.55 g (600 μL) of plant oil, a water:oil mass ratio of 0.2, and 10 mg lipase either in immobilized or free form were typically mixed in 2-mL screw-capped bottles and placed in a shaking incubator at a rate of 200 rpm and 50 °C for 24 h. Alcohol was added in three stages to prevent the accumulation of alcohol during the enzymatic reaction and consequently enzyme deactivation [25, 26]. For this propose, the total amount of alcohol was divided into three equal fractions and added at time 0 and after 5 and 10 h of the reaction. All of the above-mentioned initial conditions, as optimal conditions, were acquired in our previous study [22]. The biodiesel synthesis yields were evaluated as a function of oil type, alcohol type, alcohol:oil molar ratio, and solvent addition. To study the effect of oil and alcohol types on the reaction yield values, soybean, sunflower, canola, and palm oils were used together with methanol or ethanol with various alcohol:oil molar ratios (2:1, 3:1, 4:1, and 5:1) in the reaction systems.
The effect of the addition of n-hexane (50% v/v, based on the oil volume) into the reaction systems on the synthesis of FAME and FAEE was investigated in the transesterification reactions of palm oil with methanol and/or ethanol at various alcohol:oil molar ratios. n-Hexane is recommended as a highly intensive non-polar solvent to remove the substrate/product layer formed on the enzyme surface as it preserves the enzyme activity [27, 28].
After accomplishment of each reaction, a vacuum evaporator and centrifuge were applied to remove the residual alcohol and glycerol by-product from the reaction mixture, respectively. To determine the content of FAME and/or FAEE in the reaction mixture, first, 50 mg (~ 0.055 mL) of the remained hydrophobic phase was diluted with 1 mL of 10 mg mL−1 solution of MH in n-hexane. Then, the prepared solution was analyzed by gas chromatography (Younglin ACME6100) equipped with a TR-CN100 column (100% biscyanopropyl polysiloxane) with the fixed column temperature of 150 °C and the injector and detector temperatures of 240 °C. The area of MH peak in the GC analysis result was taken as a valid value associated with a specific amount of that material (i.e., 10 mg mL−1). The total areas of peaks in the GC spectrum of each reaction mixture, related to the synthesized FAME and FAEE were also determined. Then, the FAME and FAEE synthesis yield values, defined as the percentage of FAME and FAEE weight versus the applied oil weight, were calculated using Eq. (1).
The reusability of the immobilized lipases was investigated through repeated recovery of the immobilized lipases using a magnetic field, washing with n-hexane, and subsequently, applying into the next reaction systems consisting of fresh substrates. This procedure was repeated five times. This test was performed for transesterification of palm oil in both solvent-free and n-hexane (50% v/v) systems. The assay conditions were as the above-reported ones except alcohol:oil molar ratio which was fixed in 3:1.
Kinetics of Enzymatic Transesterification Reactions
To study the kinetics of transesterification reactions, lipase-catalyzed transesterification reactions of palm oil with methanol or ethanol were carried out at various initial concentrations of the oil (i.e., 0.2–0.7 mol L−1) and alcohol (i.e., 0.25, 0.5, 1, 1.5, and 2 mol L−1). The reaction condition was typically a water:oil mass ratio of 0.2, 10 mg of lipase either in the free or the immobilized form, reaction temperature of 50 °C, and reaction time of 2 h. Previous studies have shown that the reaction of lipase-catalyzed transesterification could be described by the Ping-Pong Bi-Bi mechanism presenting an alcohol inhibition effect [29, 30]. Therefore, this kinetic model was assumed to study the reaction kinetics. Based on this proposed mechanism, the expression for the reaction rate is given by Eq. (2):
where [A] and [B] are referred to as the concentration of oil and alcohol, respectively, V0 is the initial enzymatic reaction rate, Vmax is the maximum reaction rate, \( {K}_m^A \) and \( {K}_m^B \) are the Michaelis constants of oil and alcohol, respectively, and \( {K}_i^B \) is the inhibition constant of alcohol.
Data Analysis
Analysis of variance (ANOVA) was applied to evaluate whether or not the effects of the examined parameters are statistically significant (p < 0.05). The significance of differences was analyzed by the Duncan post hoc test. All tests were analyzed in triplicate and mean ± standard deviation values were calculated and reported.
Results and Discussion
Effects of Oil and Alcohol Types on Transesterification Reactions Yields
The results of FAME and FAEE synthesis from transesterification of palm, soybean, sunflower, and canola oils with methanol and/or ethanol in the solvent-free systems are represented in Fig. 1a, b. Regardless of the oil and alcohol type, the maximum synthesis yield values of FAME and FAEE using all of the free and immobilized lipases are obtained at 3:1 alcohol:oil molar ratio. The synthesis yield values of FAME and FAEE decrease over the further increase in the alcohol:oil molar ratios from the optimal amounts, most likely due to the fact that the excessive amount of alcohol might remove the essential water layer covering the enzymes, inhabit the enzymes surface, and consequently lead to the loss of the enzymes catalytic activity [3, 28, 31]. By considering the reaction systems operating with the similar type of oil at the similar molar ratio of alcohol:oil, it is indicated that when ethanol is applied, the synthesis yield values are significantly (p < 0.05), about 6–8.7%, higher than those obtained using methanol. According to the literature [15, 16, 28], it can be due to the fact that methanol has much more intensive deactivation effect on the enzyme molecules in comparison with ethanol. Furthermore, independent of oil and alcohol type, the maximum synthesis yield values of FAME and FAEE based on the lipase state, follow the order of L-PEI-MS > L-PAA-MS > free lipase. The results imply that the free enzyme molecules are more sensitive to the transesterification reaction conditions and lose their activity due to the denaturation [32, 33]. In the case of the immobilized enzymes, the covalent attachment of enzyme molecules to the polymer matrix can prevent them from denaturation. In our previous studies [23, 24], the results of the secondary structure analysis of the free and immobilized lipases at 50 °C confirmed the conformational stability of this kind of immobilized lipases under the applied conditions. Moreover, it can be indicated that the transesterification efficiency using the lipase immobilized onto the polymer-coated samples depends on the type of the polymer layer. Higher values of FAME and FAEE synthesis yields using L-PEI-MS in comparison with those of L-PAA-MS may be related to the hydrophobic property of the former. The hydrophilic/hydrophobic properties of the particles have been studied through determining the solvent content of the particles immersed in water, methanol, and soybean oil, in our previous work [22]. It has been revealed that the L-PEI-MS and L-PAA-MS samples have hydrophobic and hydrophilic characteristics, respectively.
Therefore, it is assumed that the L-PEI-MS system with hydrophobic property might simultaneously overcome two restrictions related to the enzymatic transesterification reactions that resulted from the immiscibility of two substrates (i.e., the mass transfer resistance against the diffusion of oil molecules towards the enzyme molecules and enzyme deactivation by methanol) [25, 28, 34]. Enhanced oil local concentration and limited methanol concentration around the L-PEI-MS sample provide a suitable condition for the reactants as well as immobilized lipases, leading to the improved high FAME and FAEE synthesis yield values. On the contrary, the hydrophilic L-PAA-MS system might encourage the absorption of water and methanol onto the particles and, hence, obstacle the diffusion of oil molecules towards the immobilized lipase molecules [21, 22]. Therefore, the lower FAME and FAEE synthesis yield values are resulted using such a biocatalyst.
Investigating the effect of the type of oil on the transesterification reactions progress using all the free and immobilized lipases shows that the FAME and FAEE synthesis yield values from palm oil are much higher than those obtained from other plant oils (Fig. 1a, b). The differences between the performance of palm oil and other oils were statistically significant (p < 0.05). The maximum FAME synthesis yield values from palm oil using free lipase, L-PAA-MS, and L-PEI-MS are 35%, 54.5%, and 81.2%, respectively. The maximum FAEE synthesis yield values of 41.8%, 62.2%, and 88.3% are also obtained from palm oil using the free lipase, L-PAA-MS, and L-PEI-MS, respectively. These results also indicate negligible differences among the FAME or FAEE synthesis yield values obtained from the transesterification of soybean, sunflower, and canola oils. In other words, the differences among the performances of those three former oils are not statistically significant (p < 0.05). Considering the physicochemical properties and fatty acid composition of plant oils (Table 1), it can be noticed that the content of fatty acids of 16 and/or 18 carbons in oils mainly causes difference among the biodiesel yield values obtained using them. For example, the higher FAME and FAEE yield values from palm oil can be attributed to its relatively high content of fatty acids of 16 carbons and consequently easier miscibility with alcohols compared with other types of oils containing fatty acids of larger carbon numbers (i.e., C18) [7].
Effect of Solvent Addition in Transesterification Reaction Media on FAME and FAEE Synthesis Yields
The transesterification of palm oil with methanol or ethanol catalyzed by the free and immobilized lipases was also carried out in the n-hexane included systems (Fig. 2a, b). By addition of n-hexane, the FAME synthesis yield values increase by 7–11.1% (p < 0.05); i.e., the free lipase, L-PAA-MS, and L-PEI-MS result in 44%, 65.6%, and 88.9% FAME synthesis yield values, respectively (Fig. 2a). However, in the reaction systems containing ethanol, addition of n-hexane leads to about 5–5.9% increase in the FAEE synthesis yield values (i.e., FAEE synthesis yield values of 47.7%, 67.3%, and 93.3% using the free lipase, L-PAA-MS and L-PEI-MS, respectively) (Fig. 2b). The enhanced FAME and FAEE synthesis yield values of the performed transesterification reactions with the addition of solvent in the media can be more likely due to three reasons: (i) the dilution of hydrophobic substrate (oil molecules) and consequently enhancement of their possible interactions with enzyme molecules, (ii) the decrease resistance of mass transfer placed between substrate components, and (iii) the decrease of the alcohol concentration around the enzymes active sites and consequently the lower deactivation of immobilized enzymes by alcohol [10, 11].
As the obtained results show, it is also indicated that the effect of solvent addition is more pronounced for the reactions with methanol in comparison with those with ethanol (Fig. 2). As mentioned previously, the deactivation effect of short-chain methanol on enzymes is higher than in relatively long-chain ethanol [15, 16, 28]; therefore, the third role of the solvent may be more effective in the systems carried out with methanol. Furthermore, similar to the solvent-free media, the order of FAME and FAEE synthesis yield values in the n-hexane systems is L-PEI-MS > L-PAA-MS > free lipase. As described above, a higher activity of the immobilized lipases compared with the free lipase is more likely to be related to their conformational stability due to covalent attachment onto the polymer layers. Also, a higher catalytic activity of L-PEI-MS in comparison with L-PAA-MS is related to the hydrophobic property of the former.
Reusability of Immobilized Lipases in Transesterification Reactions of Palm Oil
For the development of a cost-effective enzymatic process, the reusability of biocatalysts is an important parameter [1,2,3]. The reusability of the immobilized lipases through the transesterification of palm oil with optimal amounts of methanol or ethanol (i.e., alcohol:oil molar ratio of 3:1) in both solvent-free and n-hexane systems was investigated through rapid separation and recovery of the biocatalysts using a magnet and their application in new media. Figure 3 a demonstrates that relatively high values of the FAME and FAEE synthesis yield are still obtained after 5 times of recycling; L-PEI-MS and L-PAA-MS result in 28.5% and 51.4% FAME synthesis yield values, respectively, in the solvent-free systems. In the solvent-free media operated with ethanol, 36.1% and 62.1% FAEE synthesis yield values are obtained in the reactions catalyzed by L-PEI-MS and L-PAA-MS, respectively (Fig. 3b). Furthermore, when n-hexane is added to the reaction systems, the immobilized lipases can be repeatedly used over 5 consecutive cycles possessing about 7.3–13.1% higher yield values in comparison with those obtained in the solvent-free systems (p < 0.05) (i.e., FAME synthesis yield values of 39.9% and 64.5% by using L-PEI-MS and L-PAA-MS, respectively, and FAEE synthesis yield values of 43.4% and 72.6% by using L-PEI-MS and L-PAA-MS, respectively) (Fig. 3a, b). The decreases in the transesterification reactions efficiencies upon repeated uses might be due to the deactivation of enzyme molecules by the alcohol and glycerol molecules. These molecules can easily be adsorbed onto the lipases surface, and adversely affect the lipases’ activity and stability [27, 28]. However, as it can be seen, by addition of n-hexane to the reaction systems, these effects are lessened and consequently, the reaction yield values increase (Fig. 3), which is in agreement with previous reports [10, 11].
At the same reaction conditions, L-PEI-MS shows better reusability in comparison with L-PAA-MS; as described before, it can be related to the hydrophobic property of the former which limits the absorption of hydrophilic materials and consequently lessens their presence in the microenvironment of the immobilized enzyme molecules and hamper their deactivation effects.
Kinetics of Transesterification Reactions of Palm Oil with Methanol and Ethanol
Figure 4 a–f represent the results of the study of transesterification reactions kinetics. For all of the free and immobilized lipases at each given palm oil concentration, the initial reaction rates decrease with the increase in the initial alcohol concentration, fairly indicating that methanol and ethanol have considerable inhibition effect on the enzymatic reactions [35, 36]. Figure S1 shows the Lineweaver–Burk plot of reciprocal of the obtained reaction rates versus reciprocal of palm oil concentrations at various methanol or ethanol concentrations (using the same data presented in Fig. 4a–f). The four fitting straight lines in Fig. S1 are found to be unparalleled to each other, which suggests that the enzymatic reaction does not follow simple Ping-Pong Bi-Bi mechanism. Moreover, when alcohol concentration increases, the slops of the fitting straight lines are raised and the intercepts are reduced. According to the literature [37], these results agree well with the existence of substrate inhibition in lipase-catalyzed synthesis of FAME and FAEE, which implies that alcohol molecules are able to bind lipase to form a dead-end enzyme-substrate complex and, as a consequence, the formation of product molecules would be inhibited. Taking these results together, Ping-Pong Bi-Bi mechanism with alcohol inhibition (Eq. 1) is thus proposed to describe the kinetics of the reactions presented in the current work.
By taking the reciprocal of Eq. (2) and arranging terms, we obtain Eq. (3):
From Eq. (3), Eqs. (4) and (5) are derived:
where S is the slope in Eq. (3) and I is the intercept in Eq. (3).
For each reaction system, the slopes and the intercepts in Fig. 2 give the S and I values, respectively. According to Eq. (5), we can obtain \( {K}_m^B \) and Vmax values from the relation between I and 1/[B]. Furthermore, \( {K}_m^A \) and \( {K}_i^B \) values can also be determined from the relation between S and [B] according to Eq. (4). As a consequence, the kinetic parameters are calculated and listed in Table 2. As reported in Table 2, using both alcohols, the Vmax values for the immobilized lipases are much higher than those for the free lipase, revealing the improved catalytic activities of lipases through immobilization onto the polymer-coated particles. Comparing the kinetic parameters of immobilized lipases, it is revealed that the Vmax values of L-PEI-MS are 4- and 4.7-fold larger than those of L-PAA-MS in the reaction systems with methanol and ethanol, respectively. The better catalytic performance of L-PEI-MS in comparison with L-PAA-MS can be due to the hydrophobic properties of the former which provides a suitable concentration of substrates around the immobilized lipase keeping safe the conformation and catalytic properties of the lipase against the alcohols. In addition, for all the free and immobilized lipases, in the reaction systems performed with ethanol, higher Vmax values are observed as compared with those achieved in the systems operated with methanol. According to the previous studies [14,15,16], as ethanol is a relatively larger molecule, compared with methanol molecule, it is a bit more miscible with oil molecules resulting in a proper alcohol/oil concentration around the lipase which reduces the deactivating effect of ethanol on the enzyme molecules.
Considering the \( {K}_i^B \) values (Table 2), the inhibition effect of both alcohols on the lipases is in the order of L-PAA-MS > free lipase > L-PEI-MS. The lower value of \( {K}_i^B \) for L-PEI-MS verifies the hydrophobic properties of this sample preventing the deactivation of lipase by alcohol molecules to some extents. In contrast, the high affinity of hydrophilic L-PAA-MS for alcohol molecules intensifies the inhibition effect of alcohols and the formation of dead-end lipase-alcohol complexes on the immobilized lipase molecules in this system. The lower \( {K}_i^B \) values for the reaction media with ethanol as compared with those of the systems performed with methanol, as described above, can be due to the enhanced miscibility of the oil and ethanol reducing the inhibition effect of this type of alcohol on the lipase molecules. The values of \( {K}_m^A \) and \( {K}_m^B \) for the free lipase are lower than those of two immobilized lipases (Table 2). It is noted that higher values of \( {K}_m^A \) and \( {K}_m^B \) indicate lower enzyme affinity to the substrates (alcohol and oil, respectively).
According to the literature [38], the difference among the \( {K}_m^A \) values or \( {K}_m^B \) values of the free and immobilized lipases can be due to the changes in the enzyme molecules conformations during the immobilization process, affecting the enzyme molecules affinity to the substrates. The results of conformational changes in the lipase molecules upon the immobilization have been reported in our previous studies [23, 24]. The \( {K}_m^B \) value of the L-PEI-MS sample is significantly higher than that of the L-PAA-MS sample (p < 0.05). This may have been caused by the hydrophobic property of the PEI-coated particles limiting alcohol concentration around the immobilized lipases. In contrast, the L-PEI-MS sample shows a lower \( {K}_m^A \) value in comparison with the L-PAA-MS sample revealing higher affinity of the former to hydrophobic substrate (oil). The lower affinity of L-PAA-MS to oil may be due to the hydrophilic properties of the immobilization support which increases the alcohol concentration around the immobilized lipase, reducing its affinity to oil. These results appeared to verify that alcohol molecules are limited to interact with the lipase immobilized onto PEI-MS particles and form a dead-end complex, which results in higher catalytic activity of such immobilized lipase in the transesterification reactions.
Conclusions
This study dealt with the rational design of the magnetically recoverable nanobiocatalysts in order to produce biodiesel with high reaction efficiency by considering the compatibility of reactants with the physicochemical properties of the support particles used for the enzyme immobilization and studying the optimal reaction conditions. The catalytic performance of the free and immobilized lipases with significant differences, under all reaction conditions, follows the order of L-PEI-MS > L-PAA-MS > free lipase. The best performance of L-PEI-MS can be related to its hydrophobic characteristics which makes it possible to increase the oil molecules concentration around the immobilized lipases and decrease the lipases deactivation by alcohol. Among four different types of plant oils, i.e., soybean, sunflower, canola, and palm oils, the largest FAME and FAEE yield values are obtained from the transesterification of palm oil using either free lipase or immobilized lipases. In addition, irrespective of the oil type and lipase form, FAEE yield values are significantly higher than FAME ones. The addition of n-hexane in the reaction systems makes a significant increase in the transesterification reaction yields and enhances the operational stabilities of the immobilized lipases. The reactions kinetics is described by Ping-Pong Bi-Bi mechanism fairly confirming higher catalytic activity of L-PEI-MS as the inhibition effect of both alcohols on the lipases is in the order of L-PAA-MS > free lipase > L-PEI-MS. This study shows that L-PEI-MS can be recommended as a suitable nanobiocatalyst for the production of biodiesel using various plant oils.
Abbreviations
- PEI:
-
Polyethyleneimine
- PAA:
-
Polyacrylic acid
- MS:
-
Magnetic silica (Fe3O4 cluster@ SiO2) nanocomposite particles
- PEI-MS:
-
PEI-coated magnetic silica nanocomposite particles
- PAA-MS:
-
PAA-coated magnetic silica nanocomposite particles
- L-PEI-MS:
-
Lipase-immobilized PEI-coated magnetic silica nanocomposite particles
- L-PAA-MS:
-
Lipase-immobilized PAA-coated magnetic silica nanocomposite particles
- FAME:
-
Fatty acid methyl esters
- FAEE:
-
Fatty acid ethyl esters
- V 0 :
-
Initial enzymatic reaction rate (mol L−1 min−1)
- V max :
-
Maximum reaction rate (mol L−1 min−1)
- \( {K}_m^A \) :
-
Michaelis constants of oil (mol L−1)
- \( {K}_m^B \) :
-
Michaelis constants of alcohol (mol L−1)
- \( {K}_i^B \) :
-
Inhibition constant of alcohol (mol L−1)
References
Chen G, Liu J, Qi Y, Yao J, Yan B (2016) Biodiesel production using magnetic whole-cell biocatalysts by immobilization of Pseudomonas mendocina on Fe3O4-chitosan microspheres. Biochem Eng J 113:86–92
Amoah J, Ho SH, Hama S, Yoshida A, Kondo A (2016) Converting oils high in phospholipids to biodiesel using immobilized Aspergillus oryzae whole-cell biocatalysts expressing Fusarium heterosporum lipase. Biochem Eng J 105:10–15
Ying H, Zhang L, Wu D, Lei Q, Guo Y, Fang W (2017) Ionic strength-response hyperbranched polyglycerol/polyacrylic acid hydrogel for the reversible immobilization of enzyme and the synthesis of biodiesel. Energ Convers Manage 144:303–311
Pan H, Zhang L, Li X, Guo D (2017) Biosynthesis of the fatty acid isopropyl esters by engineered Escherichia coli. Enzym Microb Technol 102:49–52
Fan Y, Wang X, Zhang L, Li J, Yang L, Gao P, Zhou Z (2018) Lipase-catalyzed synthesis of biodiesel in ahydroxyl-functionalized ionic liquid. Chem Eng Res Des 132:199–207
Galeano JD, Mitchell DA, Krieger N (2017) Biodiesel production by solvent-free ethanolysis of palm oil catalyzed by fermented solids containing lipases of Burkholderia contaminans. Biochem Eng J 127:77–86
Moser BR (2011) Influence of extended storage on fuel properties of methyl esters prepared from canola, palm, soybean and sunflower oils. Renew Energy 36:1221–1226
Kareem SO, Falokun EI, Balogun SA, Akinloye OA, Omeike SO (2017) Enzymatic biodiesel production from palm oil and palm kernel oil using free lipase. Egypt J Petrol 26:635–642
Efe S, Akif Ceviz M, Temur H (2018) Comparative engine characteristics of biodiesels from hazelnut, corn, soybean, canola and sunflower oils on DI diesel engine. Renew Energy 119:142–151
Gagnon MD, Vasudevan PT (2011) Effects of solvent and enzyme source on transesterification activity. Energ Fuel 25:4669–4674
Fu B, Vasudevan PT (2009) Effect of organic solvents on enzyme-catalyzed synthesis of biodiesel. Energ Fuel 23:4105–4111
Pollardo AA, Lee H, Lee D, Kim S, Kim J (2018) Solvent effect on the enzymatic production of biodiesel from waste animal fat. J Clean Prod 185:382–388
Naranjo JC, Cordoba A, Giraldo L, Garcia VS, Moreno-Pirajan JC (2010) Lipase supported on granular activated carbon and activated carbon cloth as a catalyst in the synthesis of biodiesel fuel. J Mol Catal B Enzym 66:166–171
Verma P, Dwivedi G, Sharma MP (2017) Comprehensive analysis on potential factors of ethanol in Karanja biodiesel production and its kinetic studies. Fuel 188:586–594
Li Q, Xu J, Du W, Li Y, Liu D (2013) Ethanol as the acyl acceptor for biodiesel production. Renew Sust Energ Rev 25:742–748
Verma P, Sharma MP (2016) Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimization. Fuel 180:164–174
Liu Y, Liu T, Wang X, Xu L, Yan Y (2011) Biodiesel synthesis catalyzed by Burkholderia cenocepacia lipase supported on macroporous resin NKA in solvent-free and isooctane systems. Energ Fuel 25:1206–1212
Kalantari M, Kazemeini M, Arpanaei A (2013) Evaluation of biodiesel production using lipase immobilized on magnetic silica nanocomposite particles of various structures. Biochem Eng J 79:267–273
Noureddini H, Gao X, Philkana RS (2005) Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour Technol 96:769–777
He J, Xu Y, Ma H, Zhang Q, Evans DG, Duan X (2006) Effect of surface hydrophobicity/hydrophilicity of mesoporous supports on the activity of immobilized lipase. J Colloid Interf Sci 298:780–786
Ferrario V, Veny H, Angelis ED, Navarini L, Ebert C, Gardossi L (2013) Lipases immobilization for effective synthesis of biodiesel starting from coffee waste oils. Biomolecules 3:514–534
Esmaeilnejad-Ahranjani P, Kazemeini M, Singh G, Arpanaei A (2018) Effects of physicochemical characteristics of magnetically recoverable biocatalysts upon fatty acid methyl esters synthesis from oils. Renew Energy 116:613–622
Esmaeilnejad-Ahranjani P, Kazemeini M, Singh G, Arpanaei A (2015) Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: effects of functional molecule size on properties of the immobilized lipase. RSC Adv 5:33313–33327
Esmaeilnejad-Ahranjani P, Kazemeini M, Singh G, Arpanaei A (2016) Study of molecular conformation and activity-related properties of lipase immobilized onto core-shell structured polyacrylic acid-coated magnetic silica nanocomposite particles. Langmuir 32:3242–3252
Talukder MMR, Das P, Fang TS, Wu JC (2011) Enhanced enzymatic transesterification of palm oil to biodiesel. Biochem Eng J 55:119–122
Xie W, Ma N (2010) Enzymatic transesterification of soybean oil by using immobilized lipase on magnetic nano-particles. Biomass Bioenergy 34:890–896
Volpato RCG, Wada K, Antonio M, Ayub Z (2008) Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J Am Oil Chem Soc 85:925–930
Giraldo L, Moreno-Pira JC (2012) Lipase supported on mesoporous materials as a catalyst in the synthesis of biodiesel from Persea americana mill oil. J Mol Catal B Enzym 77:32–38
Veny H, Aroua MK, Sulaiman NMN (2014) Kinetic study of lipase catalyzed transesterification of jatropha oil in circulated batch packed bed reactor. Chem Eng J 237:123–130
Al-Zuhair S, Ling FW, Jun LS (2007) Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochem 42:951–960
Kuo CH, Peng LT, Kan SC, Liu YC, Shieh CJ (2013) Lipase-immobilized biocatalytic membranes for biodiesel production. Bioresour Technol 145:229–232
Wang YD, Shen XY, Li ZL, Li X, Wang F, Nie XA, Jiang JC (2010) Immobilized recombinant Rhizopus oryzae lipase for the production of biodiesel in solvent free system. J Mol Catal B Enzym 67:45–51
Salis A, Pinna M, Monduzzi M, Solinas V (2008) Comparison among immobilised lipases on macroporous polypropylene toward biodiesel synthesis. J Mol Catal B Enzym 54:19–26
Guldhe A, Singh P, Kumari S, Rawat I, Permaul K, Bux F (2016) Biodiesel synthesis from microalgae using immobilized Aspergillus Niger whole cell lipase biocatalyst. Renew Energy 85:1002–1010
Tokuyama H, Naito A, Kato G (2018) Transesterification of triolein with ethanol using lipase-entrapped NIPA-co-PEGMEA gel beads. React Funct Polym 126:83–86
Li Y, Du W, Dai L, Liu D (2015) Kinetic study on free lipase NS81006-catalyzed biodiesel production from soybean oil. J Mol Catal B Enzym 121:22–27
Zhang DH, Li C, Zhi GY (2013) Kinetic and thermodynamic investigation of enzymatic L-ascorbyl acetate synthesis. J Biotechnol 168:416–420
Lopresto CG, Calabro V, Woodley JM, Tufvesson P (2014) Kinetic study on the enzymatic esterification of octanoic acid and hexanol by immobilized Candida antarctica lipase B. J Mol Catal B Enzym 110:64–71
Funding
The research is financially supported by the National Institute of Genetic Engineering and Biotechnology of Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 402 kb)
Rights and permissions
About this article
Cite this article
Esmaeilnejad Ahranjani, P., Kazemeini, M. & Arpanaei, A. Green Biodiesel Production from Various Plant Oils Using Nanobiocatalysts Under Different Conditions. Bioenerg. Res. 13, 552–562 (2020). https://doi.org/10.1007/s12155-019-10022-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10022-9