Abstract
The cultivation of microalgae gained high attention within the last years because of their potential to substitute conventional bioenergy crops. To evaluate algal bioenergy production pathways already at an early stage, several life cycle assessment (LCA) studies have been performed, but their results and conclusions vary drastically. Against this background, this review gives a comparative analysis of 16 recent studies. To allow for a comparison, a meta-approach served to uniform the considered systems. System boundaries have been equalized and the energy return on investment (EROI) has been calculated for each study. Depending on the assumptions made on biomass productivity, lipid content, required energy, and the output of the system, the energetic performance was assessed. Large variations from 0.01 to 3.35 for the EROI could be derived.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are considered to be a promising feedstock for several purposes [1]. They can be used as raw material to produce high value products, e.g., for pharmaceutical applications, as fine chemicals or in human nutrition. To achieve economically viable products and to conquer bulk markets like the bioenergy sector, microalgal application has been studied in biorefinery contexts lately [2]. Microalgae can have high oil or carbohydrate contents that can be converted to liquid fuels like biodiesel or bioethanol, as well as gaseous energy carriers such as biogas (biomethane) [3]. Some algal species are even suitable to produce biohydrogen [4].
Consequently, algae-to-energy systems are considered to be a promising solution to overcome the energy vs. food dilemma, as they can be grown in absence of fertile soil in technical systems, so-called photobioreactors (PBRs). In general, algae can be produced in two different production systems open ponds or closed PBRs. Basically, the choice of the production system depends on economic-driven decisions and the final target product. If the goal is to achieve an energy product, which has a relatively low economic value, the production costs as well as the interlinked energetic efficiencies have to be considered. Therefore, it seems obvious that simple open pond systems are favorable compared to sophisticated closed PBRs. Advantages and disadvantages are described in Resurreccion et al. [5] and Tredici [6].
Production Systems
Generally, pumps are used to transfer the culture to the actual harvesting equipment. Flocculation, filtration, centrifugation, and drying are common methods to dewater and concentrate the biomass. Depending on the initial biomass concentration in the culture medium, the harvesting can have significant influence within the entire production chain [7]. After the concentration process, the biomass gets fractionated. Cells have to be disrupted and certain compounds are extracted. According to the desired product, the algae get processed. The lipid fraction is extracted for the conversion into biodiesel or other oil-based products. The residual biomass can be processed to biogas.
Algal cultivation and harvesting are important process steps within the production chain. For the whole life cycle assessment (LCA) of algal products, the consecutive steps like fractionation and downstream processing have to be included. Generally, all processes can vary enormously depending on the technology used.
Sustainability Assessment of Algae-to-Energy Systems
When assessing the sustainability of algae-to-energy systems, the essential step is to get the systems’ inputs and outputs into balance.
Consequently, the reactor systems themselves, representing the infrastructure, as well as operational materials and auxiliaries have to be assessed according to their environmental footprint. The most relevant factor for assessing the energetic efficiency is the energy to operate the production system in a reasonable configuration under proper conditions.
Based on empirical lab, pilot-scale data, and assumptions on projections made based on literature, these different algae-to-energy pathways have been assessed in several LCA studies. A comparison of their results is crucial to conclude over all resource use, environmental impacts, and trade-offs of algal bioenergy production. However, a simple comparison proved to be difficult or even misleading, because the studies differ in goal and scope, functional units, and system boundaries, but also in production assumptions and reference systems.
With this review, we aim to analyze LCA studies on microalgae-to-energy systems and identify bottlenecks within the process chains. Our intention is to update, complement, and uniform the data baseline for the comparison of the energy efficiency and comprehensive evaluation of recent studies on algae-based fuels, similar to the work that had been done previously [8,9,10].
Motivation
Focusing on microalgal production for energy application, data given for energy inputs and outputs are essential to derive one of the applied comparison measures, the energy return on investment (EROI) indicator. In this review, 16 studies were selected due to their relevance (citation impact) and data availability on the main process steps. According to this, results from previous meta-analysis studies were included in the comparison to draw a substantial and conclusive picture.
The data quality highly depends on the considered production scale. Most studies work on projected pilot scale which goes along with high uncertainties. Additionally, debatable assumptions strongly influence the results and the recommended guidelines for LCA, like the ILCD handbook, are interpreted unconventionally as in [11]. Most of the reviewed studies focus on criteria such as energy balance or greenhouse gas (GHG) emissions. This might be due to the focus on energy production pathways and the large scientific and political interest on these criteria, which are essential to evaluate microalgal contribution to a sustainable energy supply. Nevertheless, it should be taken into consideration that other impact categories, such as land occupation, might also prove relevant for decision making processes in science, politics, and economy.
We present a comprehensive overview for an LCA meta-study with the calculation of the EROI on algal energy products and provide a basic understanding of the challenges arising through conducting LCAs at an early stage of the technology development process. This review was carried out within the EnAlgae project, funded by the INTERREG IVB NWE Programme.
Methodology
For a credible comparison of selected recent LCA studies (2009 to 2015), the first step was to identify the functional unit. Due to the variety of functional units, on the one hand related to the produced mass (e.g. kg of dry biomass), and on the other hand to the energy content (e.g. MJ of biodiesel), the results of the reviewed studies could not be compared directly. Therefore, the functional units have been converted to the “meta-unit” of 1 kg of dry algal biomass to allow for a reference of inputs and outputs on the same baseline. Nevertheless, one has to keep in mind that several factors are influencing the energy input and output, such as nitrogen limitation leading to an accumulation of triacylglycerides (TAGs). An increased TAG content per kilogram of biomass results in a higher energy content [3], leading to relatively increased energy returns on invested energy. Therefore, the focus of the examined studies was mostly on the production of biodiesel.
Meta-System
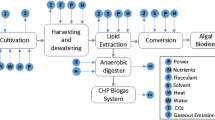
For a better comparison of the microalgal LCA studies, the results were distinguished by the type of the production system (open pond or closed PBR). The vast majority of the studies have assessed open pond production systems; only few have tested closed PBRs (see Table 2). The different system boundaries have been equalized and a meta-system has been developed to guarantee an adequate comparison. A scheme of the considered production steps is shown in Fig. 1. Direct inputs to the production like pumping electricity or fertilizer have been included, whereas the prevailing infrastructure contributions and the footprint of the construction materials remained unconsidered, because of their variability, reported in the original studies.
According to ISO 14040:2006 [12], the processes included in the system shall refer to the goal and scope definition. In case of the reviewed studies, relevant processes, which cause resource/energy use and GHG emissions, were addressed: the production of chemicals (e.g. fertilizer) and process energy, cultivation of algal biomass feedstock, and conversion to an energy carrier (e.g. biodiesel also including transport steps up to the final combustion in an engine). The definition of the system boundaries strongly influences the results of the studies. Consequently, there can be a large variation in environmental performance due to a different choice of system boundaries. Although the ISO 14044:2006 directive is a framework for performing comprehensive LCAs, still many decisions, boundaries, and assumptions remain subjective and may sometimes be debatable.
Data of all studies could be obtained for the biomass production process. Most studies produced the liquid energy carrier biodiesel. If the downstream process towards biodiesel was not chosen originally, a generic downstream module was added for the oil extraction and transesterification process based on data derived by Lardon et al. [13]. Another restriction on direct comparability was the handling of by-products and allocation as well as credits granted. To keep conformity between the systems, by-products and credits were not taken into account. Therefore, all inputs were allocated to one single energy output. Some examples of systems which needed to be adapted are listed in the following section.
In Jorquera et al. [14], highly optimistic assumptions are made concerning energy requirements, which are covered by hydropower resulting in hardly any environmental burden. Studies mainly differ in their scope of modeling the production chain according to the individual demands. For instance, Sevoigné Itoiz et al. [15] limit their system to the step of biomass production. Lardon et al. [13] and Collet et al. [16] also include the biomass pre-treatment processes; the conversion to the energy carrier, in this case biodiesel and biogas; and the final combustion in a diesel engine or the application of the biogas combustion. Yanfen et al. [17] additionally include the distribution of the fuel to the supply stations.
The results were discussed against the background of the different system boundaries and assumptions made in the reviewed studies. The most important reasons for differences in the results were highlighted. For the illustration of the results, three main process steps were clustered. Table 1 shows the original data provided and processes added to sustain equal system boundaries.
Calculation of the Energy Return on Investment
For the comparison of the energy demand, the EROI has been calculated as an indicator for the energy efficiency of a system, process, or product following the approach of Mulder & Hagens [27]:
and
Depending on the definition, this value is sometimes also called net energy ratio (NER) [28].
The product energy output has the unit MJ kg−1 and refers to the energetic fraction of algal biomass used to produce the final energy product (e.g. lipids form biodiesel production) related to 1 kg of dry algal biomass:
The primary energy input has the unit MJ kg−1 and relates to 1 kg of dry algal biomass. Baseline information about the conversion of the primary energy can be found in the supplementary information. For this analysis, all direct energy inputs within the considered process chain, which have been included in the reviewed studies, were converted to a primary energy input following the ecoinvent database (version 3.01). Furthermore, the cumulative energy demand (CED) relating to the nutrient demand has been taken into account and added to the primary energy input using the ecoinvent database (version 3.01)Footnote 1 again, if it was not included in the dataset given in the study yet. The nutrient demand (nitrogen and phosphorus) was recalculated and expressed as gram per kilogram dry algal biomass (see supplementary information), as fertilizer production was considered to contribute to the CED significantly as for conventional crops [29]. The CED resulting from material inputs other than fertilizer, which have not been included in the original studies, has not been taken into account in our EROI calculations. If not indicated specifically (see Table 1), an oil recovery and conversion efficiency/product conversion efficiency of 100% was applied.
The value of the EROI refers to the product output. An EROI value greater than 1 indicates that the energy output is higher than the input and that the net energy balance is positive. Correspondingly, values lower than 1 refer to a negative energy balance. Since the primary energy input is the sum of the inputs of all involved processes, the EROI of the studies focusing at different products is not strictly comparable, as different downstream processes were involved.
Results and Discussion of the Reviewed Studies
In the following section, the results of the analyses and comparisons of the 16 reviewed microalgal LCA studies are presented and discussed, providing a comprehensive overview on bioenergy production from microalgae. The main goal of the selected studies was to provide baseline information about microalgal energy production, to evaluate their feasibility and identify obstacles and limitations for future research steps. The studies focused on comparing the environmental burden of different production processes and techniques within the cultivation. Different kinds of energy products were addressed, such as biodiesel or methanol. As a consequence, differences arose regarding biomass production pathways and final biomass composition. According to the target product, production processes, nutrient supply, and the choice of suitable algal strains can differ significantly. Besides, the production systems among the reviewed studies varied concerning the use of technical equipment and material application.
Most of the reviewed studies refer to data from research (lab scale) or pilot plants because there are no commercial systems for large-scale algal fuel production in operation so far. Due to the small-scale and immature technology, data on performance, time relation, and coverage often cannot be included. A proposal on how to proceed and harmonize a LCA system at this stage of technology development is given by Bradley et al. [30]. Many authors do not use own empirical data for their LCA approach, but rely at least partly on previous publications and theoretical calculations.
EROI Comparison
The energy balance is one measure to evaluate the energy efficiency of the microalgal production. Consequently, energy outputs and inputs must be balanced. In order to produce an algal energy product, a positive energy balance is essential and an EROI greater than 1 should be achieved. The energy inputs refer to the direct inputs for the production process, recalculated to primary energy input (see Table 2).
To improve the comparability of the studies, they were aligned by adding the energy footprint of the fertilizer production, if necessary (and possible). Beside these absolute values given, the relative contribution to the EROI value is indicated in Fig. 2. Mostly, the cultivation step represents a high share in the overall EROI, followed by the downstream processing. The smallest contribution was investigated for the harvesting step.
The EROI provides information about the efficiency of the algal production system. In general, huge variations have been demonstrated. Most studies provide data about energy inputs directly related to the algal production. For open systems, a favorable EROI could be expected in comparison to closed PBRs, because of rather low influencing parameter control and, consequently, low energy input, as long as the biomass productivity remains at the same level [6].
-
Campbell et al. [18] achieved energy flows leading to an EROI of 3.35. Their assumed areal biomass yield of 109.6 t ha−1 a−1 resulted in a favorable EROI. Another reason for the high EROI value was the use of chemical flocculants combined with a flotation device as a pre-concentration step in the harvesting process. This reduced the energy demand for the following centrifugation drastically. Even though energy savings might be achieved, flocculants can have huge environmental impacts. They can influence the pH value of the culture media and might require a wastewater treatment [31]. Apart from that, flocculants are quite expensive and not applicable in large-scale algal production from an economic point of view [31, 32].
-
Clarens et al. [19] describe energy inputs leading to an EROI of the bioenergy of 0.68. Similar to Campbell et al. [18], they utilized flocculants plus centrifugation to guarantee an efficient harvesting process. They argued that 50% of the energy as well as the GHG emissions have been associated with the production of chemical fertilizers. The authors suggested covering the demand of nutrients by using wastewater, which could significantly reduce the supply of chemical fertilizers. Another main energy burden was related to the production of the supplied CO2 produced by steam reforming hydrocarbons. Summarizing, upstream processes were the main drivers for primary energy consumption in this study.
-
Collet et al. [16] assumed low fertilizer inputs (see supplementary information) because they considered a recycling of liquid digestate from an anaerobic digestion step. Low amounts of fertilizer resulted in low embedded energy, meaning a small share of the energy input for the EROI. The contribution of fertilizer to the total energy inputs accounted for 11.2%. Without this recycling step, the fertilizers and their energy footprint would have been higher by a factor of 10, further reducing the EROI value. An optimization of the underlying system was described to be potentially possible.
-
Jorquera et al. [14] originally reported very high EROI values for the algal biomass: 7.01 in open ponds and 4.33 in PBR. Originally, an energy-efficient system with hydropower as primary energy input and hardly any energy losses was described. After the system boundary adaptation, fertilizer production as well as a harvesting step were included in our meta-analysis (EROI 1.01 and 0.64). If additionally a standard electricity mix (according to ecoinvent version 3.01) was applied, the EROI was calculated to be 0.74 and 0.29.
-
Khoo et al. [20] demonstrated the high energy shares to be consumed during inoculation in PBRs. In the meta-analysis, the inoculation phase was neglected. However, the unfavorable EROI (0.71) does include energy requirements for lipid extraction, which was discussed to have a significant influence on the total energy requirements. They reported that 85% of the direct energy inputs are related to lipid extraction; still, this value does not relate to primary energy, but to electricity consumption.
-
Lardon et al. [13] reported a consumption of about 80% of energy inputs for the step of biomass drying in both scenarios (low nitrogen (N) and normal N). Still, it could be proved that the low-nitrogen (N) scenario is more energy efficient (EROI 0.50) compared to second scenario with sufficient N supply (EROI 0.20).
Nonetheless, they discuss the potential of solar drying, which seems to be one feasible method to reduce the consumption of energy, although further investigations on lipid stability and lipid decay by sunlight are essential.
Moreover, they describe a wet extraction scenario also stating that, however this approach seems promising, the data used to estimate impacts and mass flows are questionable.
-
Liu & Ma [11] analyzed a system resulting in an EROI of 2.01 which can be explained by solar drying of the algal biomass, albeit their assumptions are incoherent.
-
Razon & Tan [21] described two different production systems (scenarios), including different technologies as well as species. Hence, different EROI values could be derived. The most important contributors to energy inputs were the bead mill for algal cell disruption (scenario 1, EROI 0.18) and the heat for the algal biomass drying step (scenario 2, EROI 0.04), respectively. The bead mill in the wet extraction scenario for Haematocuoccus pluvialis accounts for 32% of the primary energy inputs. The heat used in the dry extraction scenario and Nannochloropsis contributes with 48% to the total primary energy input.
-
Sander & Murthy [22] derived energy flows leading to an EROI of 0.19. In this study, solely wastewater was assumed to cover the nutrient demand of the algal culture. The credits from the co-product allocation, which was considered to be the substitution of corn-derived ethanol, were excluded from the EROI calculation, because they would have led to a negative energy input value, which is not defined.
-
The study of Stephenson et al. [23] considered two different scenarios: open pond cultivation and algal production in PBRs, although detailed data was just provided for the open pond cultivation. A two-stage approach was performed in both cases producing biodiesel and biogas as co-product from the residuals. In the first stage, sufficient nitrogen was supplied to achieve high biomass concentrations. During the second stage, the culture was grown under nitrogen starvation conditions, which leads to an accumulation of lipids. The energy output was calculated referring to the lipid fraction of the algal biomass an oil extraction efficiency of 99% was assumed. This led to an EROI of 0.53.
-
Yanfen et al. [17] analyzed a two-stage microalgal production similar to Stephenson et al. [23]. They demonstrated high-energy requirements for algal biomass drying. This step accounted for about 80% of primary energy inputs, if the anaerobic digestion credit was neglected. Without this credit, the primary energy input is 46.89 MJ/kg. According to the numbers given, an EROI of 0.26 was calculated. They suggest an internal use of biogas generated by anaerobic digestion of microalgal residues, which could offset over 84% of the heat burden associated with the drying process.
-
In Resurreccion et al. [5], four different scenarios are described with either open pond (OP) or closed PBR systems in combination with freshwater (FW) and brackish saline water (BSW), respectively. Detailed information was provided about energy consumptions. The BSW systems perform more efficient, as higher biomass and lipid yields could be observed compared to the analogous FW system. If the infrastructure was part of the considered system, the OP systems would be favorable, especially the OP-BSW system, for which an EROI greater than 1 was reported. As the infrastructure and its CED were neglected for this meta-analysis, the total primary energy inputs are lower for the closed PBR systems (in contrast to the original study). In contrary to that, we derived EROI values of 0.09 for OP-FW, 0.15 for OP-BSW, 0.24 for PBR-FW, and 0.40 for PBR-BSW.
-
Frank et al. [24] describe a renewable diesel production with a hydrothermal liquefaction (HTL) processing step. They conclude that HTL can produce enough heat and electricity from biomass residuals via catalytic hydrothermal gasification to accomplish microalgal growth and harvesting. If HTL yields exceed a value of 0.40 g HTL oil per g algae (ash free basis), however, insufficient heat and electricity is produced on site and additional energy is required. They claim electricity reductions during cultivation to take advantage of the higher oil yield, even though they see a conflict in recycling nutrients while at the same time producing electricity and heat. In the underlying meta-analysis, an EROI of 1.78 was calculated.
-
Bennion et al. [25] studied alternative processing technologies for processing microalgae as a feedstock into biofuels. Consequently, the microalgal biofuel production was based either on HTL or pyrolysis. In the meta-analysis, the HTL process was considered. Assumptions and optimizations in terms of energy recovery and yields were derived from the experimental data. Based on the data given for industrial-scale application, an EROI value of 0.75 was calculated.
-
Sevigné Itoiz et al. [15] used an algal species which is hardly described in the literature, especially in the context of energy production. They used lab data for their calculations resulting in a very low EROI of 0.01. Although the net energy balance was negative, potential improvements were suggested, e.g., investments towards a reduction of energy inputs during the operation of the bubble-column reactor, but also more efficient construction materials with a low-energy footprint. Further investigations on co-products and associated allocation credits were described to be necessary to improve the net energy balance. The fertilizer energy footprint was replaced as the original values seemed incomprehensibly high (90.63 MJ/kg for KNO3).
-
Tredici et al. [26] described a closed PBR system. Even if the infrastructure was not considered, an unfavorable EROI was derived (0.74). They conclude that suitable climatic conditions and modifications in the reactor setup could lead to a significant improvement of the net energy balance. Additionally, they promote the research of a marine nitrogen fixing cyanobacteria strain to overcome the problem of a huge water footprint.
Discussion
It can be noticed that poor EROI results were calculated for most studies. For an energy product, achieving a positive net energy balance, an EROI greater than 1, is an absolute prerequisite [33]. Favorable values could be derived if more optimistic assumptions were made for either energy inputs or outputs.
However, studies vary strongly concerning the shares of contribution for energy consumption like for fertilizer or CO2 supply [8, 30], or for downstream processes. Differences could be analyzed due to the use of waste sources like wastewater or flue gas, for nutrient supply, and CO2 source. Embedded energy of materials should be taken into account and their material application should be optimized, especially for large-scale production [15]. Large variations to the presented results could be observed for Resurreccion et al. [5] due to the different system boundary definition. Handler et al. [10] struggled with the same problem of inconsistent data and information given, when they developed their meta-study. Not all the authors listed in their study considered the same information as mentioned above. They claim for a uniform approach in order to “accurately characterize the emerging industry of algal biofuel production.” However, comparing the primary energy inputs of Handler et al. [10] to our calculated values, the numbers given in the underlying study are significantly higher (indicated in Table 2).
Even though strong variations within the EROI results exist, the same trend towards a negative net energy balance was observed. Another literature review study performed by Slade & Bauen [9] already showed the poor energetic performance of the microalgal production systems in a similar meta-approach; however, they also emphasize that the economic and environmental impact have to be considered as well, in order to draw a holistic picture of sustainability. Only considering the energy balance, Slade & Bauen [9] state that the achievement of net energy positive production system “will require technological advances and highly optimized production systems.”
Liu et al. [8] achieved more promising results regarding the EROI. Derived from their meta-model, they infer that algae-to-energy systems might perform (energy) efficiently and even comparable to conventional biofuel production systems. Vasudevan et al. [34] calculated a NER of 0.3 if drying was included, and 2.5 if wet extraction is applied in the downstreaming to produce biodiesel (after an open pond cultivation). This is in line with the EROI results of our meta-approach and provides therefore evidence for our comprehensive approach. So far, many systems described only refer to literature data rather than on empirical numbers; therefore, large uncertainties are expected. Assumptions made on energy inputs as well as productivities can be drivers for larger intervals of results like in Campbell et al. [18]. Additionally, biomass production systems and related primary energy inputs influence the EROI results [14]. Nevertheless, most studies describe optimization potential. Being an immature technology, algal farming might be further optimized not only concerning biomass/lipid yields but also regarding energy efficiency of the overall production system.
In summary, bottlenecks concerning energy inputs could be identified during every step of microalgal fuel production (see process flow scheme in Fig. 1), depending on the process engineering approach including the direct energy inputs for pumping, harvesting, downstream processing, but also on resource use, i.e., waste stream supply. Product energy outputs were highly dependent on assumptions made on productivity resulting from different operating procedures, like growth under nitrogen starvation, as well as on conversion efficiencies.
Apart from the investigated impact categories chosen, other impacts like the water footprint of microalgal production systems would be of high interest, too. Water consumption is analyzed in few cases only, resulting in insufficient data for a comparison [17, 21]. Despite of that, when algal bioenergy is produced, the impact category land occupation should be one main criterion to be respected and compared to the land occupation of conventional energy crops [19].
Conclusions
The broad variation of the EROI from 0.01 to 3.35 for the reviewed microalgal studies indicates a high uncertainty whether algae can be a sustainable feedstock for energy carrier production. Values greater than 1 for the EROI were achieved due to highly optimistic assumptions regarding algal productivities or energy inputs. From a biological point of view, the EROI can only be improved by an efficient photo-conversion, which would lead to higher biomass yields and thus energy contents. Technically, the EROI for algal fuels can be improved by further technological progress in cultivation and harvesting systems (less material, less energy for pumping, harvesting, and drying), as well as in nutrient recycling and advanced downstream processing technologies, e.g., for fractionation. Since some of the existing studies lack transparency and comprehensibility, these should be paid special attention to in future studies. Besides, we consider impact categories like land occupation, water consumption, and fertilizer inputs as very important. The comparison of results from LCA on algal fuels can be improved by a more coherent and transparent definition of the relevant metrics such as the boundary conditions, functional units, and impact categories. Moreover, it seems worth striving for a common agreement on how to conduct such LCAs or to agree upon a harmonized approach: Bradley et al. [30] have made a proposal for a unified approach to LCAs between three unique algal biofuel facilities. By doing so, there will be no further need for a meta-analysis in order to compare LCA results.
Notes
More information on the CED values used in this analysis can be found in the supplementary information.
References
Subhadra BG (2010) Sustainability of algal biofuel production using integrated renewable energy park (IREP) and algal biorefinery approach. Energy Policy Elsevier 38:5892–5901. https://doi.org/10.1016/j.enpol.2010.05.043
Dechema-Fachgruppe “Algenbiotechnologie.” (2016) Mikroalgen-Biotechnologie Gegenwärtiger Stand, Herausforderungen, Ziele
Williams PJ l B, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3:554. https://doi.org/10.1039/b924978h
Shaishav S, Satyendra T, Singh R (2013) Biohydrogen from algae: fuel of the future. Int Res J Environ Sci 2:44–47
Resurreccion EP, Colosi LM, White M a, Clarens AF (2012) Comparison of algae cultivation methods for bioenergy production using a combined life cycle assessment and life cycle costing approach. Bioresour Technol Elsevier Ltd 126:298–306. https://doi.org/10.1016/j.biortech.2012.09.038
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162. https://doi.org/10.4155/bfs.09.10
Gerardo ML, Oatley-Radcliffe DL, Lovitt RW (2014) Minimizing the energy requirement of dewatering Scenedesmus sp. by microfiltration: performance, costs, and feasibility. Environ Sci Technol 48:845–853. https://doi.org/10.1021/es4051567
Liu X, Clarens AF, Colosi LM (2012) Algae biodiesel has potential despite inconclusive results to date. Bioresour Technol Elsevier Ltd 104:803–806. https://doi.org/10.1016/j.biortech.2011.10.077
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy Elsevier Ltd 44:1–10. https://doi.org/10.1016/j.biombioe.2012.12.019
Handler RM, Canter CE, Kalnes TN, Lupton FS, Kholiqov O, Shonnard DR et al (2012) Evaluation of environmental impacts from microalgae cultivation in open-air raceway ponds: analysis of the prior literature and investigation of wide variance in predicted impacts. Algal Res 1:83–92. https://doi.org/10.1016/j.algal.2012.02.003
Liu J, Ma X (2009) The analysis on energy and environmental impacts of microalgae-based fuel methanol in China. Energ Policy 37:1479–1488. https://doi.org/10.1016/j.enpol.2008.12.010
International Organization for Standardization. (2006) ISO 14040: environmental management—life cycle assessment—principles and framework; 2006
Lardon L, Hélias A, Sialve B (2009) Life-cycle assessment of biodiesel production from microalgae. Foreign Policy Anal:6475–6481
Jorquera O, Kiperstok A, Sales EA, Embiruçu M, Ghirardi ML (2010) Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresource Technology. Elsevier Ltd 101:1406–1413. https://doi.org/10.1016/j.biortech.2009.09.038
Sevigné Itoiz E, Fuentes-Grünewald C, Gasol CM, Garcés E, Alacid E, Rossi S et al (2012) Energy balance and environmental impact analysis of marine microalgal biomass production for biodiesel generation in a photobioreactor pilot plant. Biomass Bioenergy 39:324–335. https://doi.org/10.1016/j.biombioe.2012.01.026
Collet P, Hélias A, Lardon L, Ras M, Goy R-A, Steyer J-P (2011) Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour Technol Elsevier Ltd 102:207–214. https://doi.org/10.1016/j.biortech.2010.06.154
Yanfen L, Zehao H, Xiaoqian M (2012) Energy analysis and environmental impacts of microalgal biodiesel in China. Energ Policy 45:142–151. https://doi.org/10.1016/j.enpol.2012.02.007
Campbell PK, Beer T, Batten D (2011) Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour Technol Elsevier Ltd 102:50–56. https://doi.org/10.1016/j.biortech.2010.06.048
Clarens AF, Resurreccion EP, White M a, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819. https://doi.org/10.1021/es902838n
Khoo HH, Sharratt PN, Das P, Balasubramanian RK, Naraharisetti PK, Shaik S (2011) Life cycle energy and CO2 analysis of microalgae-to-biodiesel: preliminary results and comparisons. Bioresour Technol Elsevier Ltd 102:5800–5807. https://doi.org/10.1016/j.biortech.2011.02.055
Razon LF, Tan RR (2011) Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl Energy Elsevier Ltd 88:3507–3514. https://doi.org/10.1016/j.apenergy.2010.12.052
Sander K, Murthy GS (2010) Life cycle analysis of algae biodiesel. Int J Life Cycle Assess 15:704–714. https://doi.org/10.1007/s11367-010-0194-1
Stephenson AL, Kazamia E, Dennis JS, Howe CJ, Scott S a, Smith AG (2010) Life-cycle assessment of potential algal biodiesel production in the United Kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy Fuel 24:4062–4077. https://doi.org/10.1021/ef1003123
Frank ED, Elgowainy A, Han J, Wang Z (2012) Life cycle comparison of hydrothermal liquefaction and lipid extraction pathways to renewable diesel from algae. Mitig Adapt Strateg Glob Chang 18:137–158. https://doi.org/10.1007/s11027-012-9395-1
Bennion EP, Ginosar DM, Moses J, Agblevor F, Quinn JC (2015) Life cycle assessment of microalgae to biofuel: comparison of thermochemical processing pathways. Appl Energy Elsevier Ltd 154:1062–1071. https://doi.org/10.1016/j.apenergy.2014.12.009
Tredici MR, Bassi N, Prussi M, Biondi N, Rodolfi L, Chini Zittelli G et al (2015) Energy balance of algal biomass production in a 1-ha “Green Wall panel” plant: how to produce algal biomass in a closed reactor achieving a high net energy ratio. Appl Energy Elsevier Ltd 154:1103–1111. https://doi.org/10.1016/j.apenergy.2015.01.086
Mulder K, Hagens NJ (2008) Energy return on investment: toward a consistent framework. Ambio 37:74–79. https://doi.org/10.1579/0044-7447(2008)37[74:EROITA]2.0.CO;2
Keoleian GA, Spitzley DV (2006) Life cycle based sustainability metrics. In: Abraham MA (ed) Sustainability Science and Engineering - Defining Principles, 1at ed. Elsevier B.V, pp 127–160
Kim S, Dale B (2003) Cumulative energy and global warming impact from the production of biomass for biobased products. J Ind Ecol 7:147–162. https://doi.org/10.1162/108819803323059442
Bradley T, Maga D, Antón S (2015) Unified approach to life cycle assessment between three unique algae biofuel facilities. Appl Energy. https://doi.org/10.1016/j.apenergy.2014.12.087
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresourc Technol Elsevier Ltd 102:35–42. https://doi.org/10.1016/j.biortech.2010.06.158
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C et al (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. BioEnergy Res 1:20–43. https://doi.org/10.1007/s12155-008-9008-8
Sills DL, Paramita V, Franke MJ, Johnson MC, Akabas TM, Greene CH et al (2013) Quantitative uncertainty analysis of life cycle assessment for algal biofuel production. Environ Sci Technol 47:687–694. https://doi.org/10.1021/es3029236
Vasudevan V, Stratton RW, Pearlson MN, Jersey GR, Beyene AG, Weissman JC et al (2012) Environmental performance of algal biofuel technology options. Environ Sci Technol 46:2451–2459. https://doi.org/10.1021/es2026399
Acknowledgements
The authors gratefully acknowledge financial support by the project EnAlgae, a Strategic Initiative of the INTERREG IVB NWE Programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• System boundaries and processes were adapted in a meta-analysis
• Comparison of energy return on investment (EROI) was enabled
• Differences of results from 16 microalgal LCA studies, focusing on bioenergy, were identified
• Explanations for varying energy inputs and outputs were given and discussed
• Bottlenecks in algae-to-energy systems are summarized
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Ketzer, F., Skarka, J. & Rösch, C. Critical Review of Microalgae LCA Studies for Bioenergy Production. Bioenerg. Res. 11, 95–105 (2018). https://doi.org/10.1007/s12155-017-9880-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-017-9880-1