Abstract
Pumice, a natural porous silica material, exchanged with potassium is an efficient heterogeneous particulate catalytic material for triglycerides and free fatty acids transesterification reaction from sunflower oil and waste frying oil at low temperature. In this work, a packed-bed catalytic configuration reactor using this catalytic material was developed for biodiesel fuel production from sunflower oil and frying oil feedstock. Reactor operation variables as methanol/oil molar ratio, catalyst amount, reaction time, and reaction temperature were studied. Results were compared with those obtained from the same transesterification reaction proceeding in a slurry batch reactor. The packed-bed catalytic reactor configuration can be useful in order to minimize catalyst mechanical damage occurring in the slurry reactor due to continuous stirring. The possibility of using a packed-bed reactor shows some advantages because the catalyst stays confined in the reactor bed and the reaction products can be easily separated, besides the mechanical stability of the catalyst particles is achieved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel, defined as mono-alkyl esters of vegetable oil or animal fat, is environmentally attractive as conventional petroleum diesel fuel substitute. Produced by oil transesterification reaction, biodiesel offers a number of important technical advantages over petrodiesel. Benefits derived from its use include inherent lubricity, low toxicity, superior flash point and biodegradability, negligible sulfur content, lower exhaust emissions, and renewable and domestic feedstock utilization [1].
Commonly, biodiesel has been industrially manufactured by homogeneous catalyzed vegetable oil transesterification reaction using NaOH or KOH as basic catalyst [2–4]. Base-catalyzed transesterification reaction is much faster than acid-catalyzed transesterification reaction. Although homogeneous base catalysts show fast reaction rates under mild reaction conditions, it is difficult to remove them from reaction mixture or products. In addition, large amounts of water are needed to wash the biodiesel product. Homogeneous catalysts also are difficult to separate from glycerol generated as byproduct [5–7]. Besides, when waste frying oils are processed as feedstock, they usually contain large amount of free fatty acids (FFA) that could not be converted into biodiesel using alkaline homogeneous catalysts due to fatty acid salts (soap) formation problem. In addition to high FFA content, excess moisture (water) in waste oils is also known to interfere with alkali-catalyzed transesterification reactions, usually leading to the formation of soaps.
Heterogeneous solid base catalysts can solve these problems: they can be easily separated from the reaction mixture without the use of water as cleaning agent, they can be regenerated and reused, and they have a less corrosive character, leading to safer, cheaper, and more environment-friendly biodiesel production operations [2, 8]. During the last years, few reports have been published about biodiesel production from waste frying oil using one-step basic treatment; usually, the two-step process is used to obtain biodiesel from that environment-friendly feedstock: acid pretreatment and alkaline-catalyzed transesterification process. In order to improve that process, the production of biodiesel from high acid value feedstock needs esterification and transesterification processes occurring simultaneously.
In this paper, potassium-loaded pumice material was used as heterogeneous catalyst in sunflower oil and waste oil transesterification reaction for biodiesel production using a packed-bed catalytic reactor in a recirculating system. Pumice is an amorphous porous volcanic rock composed mainly by silica and alumina. Furthermore, pumice is a low-cost natural material, having potential applications as carrier for supported catalysts [9–12].
This particulate heterogeneous catalytic material can be used to configure a packed-bed catalytic reactor for biodiesel fuel production, avoiding slurry reactor operation-related problems. Therefore, a catalytic packed-bed reactor configuration in a recirculating system was developed and studied in order to optimize the operation variables.
There are not many studies in the literature related to mass transfer limitations on transesterification reaction rates. Both a system based on a catalytic packed-bed reactor and a slurry batch reactor were used to demonstrate any effect of the limited miscibility and mass transfer limitation on the vegetable oil transesterification reaction. Although mechanical stirring increases the reaction rate, it is associated with a decrease of the catalyst lifetime [13, 14]. Therefore, the possibility of using a packed-bed catalytic reactor minimizing catalyst mechanical damage can be interesting [15]. The aim of the catalytic packed-bed reactor study presented in this work is not to provide continuous flow-type data for engineering purposes, but to show any potential reaction rate decrease when a packed-bed reactor configuration is used compared with using a slurry batch reactor for vegetable oil transesterification reaction.
Due to the nature of waste cooking oil, which is a complex mixture of many compounds including FFA and water, the study of the heterogeneous catalyst activity in the presence of existent impurities in waste oil is important and it needs to be investigated. The purpose of this work was to develop a new clean and practical one-step heterogeneously catalyzed transesterification process with the capability of treating a wide range of feedstock.
Experimental
Pumice particles (1.40–3.0 mm) were introduced into a KOH aqueous solution in order to get a potassium interchange, creating some basic sites on the natural material. Firstly, pumice was dried in an oven in order to remove the absorbed water on the surface. Ionic exchange was carried out with a 1-M KOH solution for 24 h, and then pumice granules were dried at 120 °C for 3 h, obtaining the potassium-loaded catalytic material (K-Pumice) [16].
The synthesis of fatty acid methyl esters (FAME) or biodiesel production from sunflower oil/waste oil and methanol was carried out both in a slurry reactor configuration and in a packed-bed catalytic reactor configuration with K-Pumice as heterogeneous catalyst. The main properties of feedstock oils used as reagents are shown in Table 1.
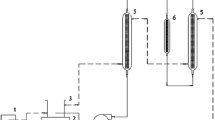
Figure 1 shows a schematic diagram of the packed-bed catalytic reactor used. Reagents were placed in a heated tank, mixed by mechanical stirring, and then fed into the packed-bed catalytic reactor using a peristaltic pump at a constant flow rate of 15 ml/min. The feed solution was supplied to the bottom of the cylindrical reactor (5 cm inner diameter and 18 cm length) packed with K-Pumice catalyst particles (1.4–3.0 mm). Catalytic bed length can be directly correlated with the catalyst weight into the reactor: h = 0.124W c + 1.981, where h is the bed length in centimeters and W c is the catalyst weight in grams.
The bed porosity (ε b = 0.74) was determined using the following equation [17]:
where ρ c is the density of the catalyst and d is the inner diameter of the reactor.
Methanol/oil molar ratio, catalyst amount, reaction time, and reaction temperature were varied in order to investigate the optimum operation conditions of the transesterification reaction. After completion of the reaction or stipulated reaction time, the product obtained was rotary evaporated to remove the remaining methanol and then settled in a separating funnel: the upper phase containing FAME (biodiesel product) and the lower phase containing glycerol by-product. Viscosity and density of the biodiesel product were measured using a rotational viscometer VISCO STAR Plus L and a pycnometer, respectively. Moreover, the biodiesel product obtained was analyzed by 1H nuclear magnetic resonance spectrometry in order to estimate the FAME yield [18, 19].
Results and Discussion
K-Pumice activity has been tested in a previous work, showing it to be more active than pumice support material [16]. Chemical and textural properties of both materials have been studied in that previous work and temperature-programmed desorption studies showed the presence of acid and basic surface sites widely distributed in K-Pumice catalyst. Therefore, this bifunctional catalyst presented high activity when oils with high FFA content were used as feedstock due to the presence of acid sites on the catalyst surface capable of carrying out the FFA esterification reaction, thus preventing soaps formation.
In this paper, the K-Pumice catalytic activity was tested in the sunflower oil and waste frying oil transesterification reaction using a packed-bed catalytic reactor. The aim of this work is to study the packed-bed catalytic reactor system performance, comparing it with those results obtained from a slurry batch reactor system under the same operation conditions. Operation variables such as methanol/oil molar ratio, catalyst amount, reaction time, and reaction temperature were studied in order to achieve the maximum catalytic activity.
The vegetable oil transesterification reaction requires a methanol/oil molar ratio higher than the stoichiometric ratio in order to drive the reaction towards completion, producing more methyl esters as product [7]. As can be seen in Fig. 2, the methanol/oil molar ratio variable has a significant impact on the FAME yield in the biodiesel product obtained. It can be observed that, when the methanol/oil molar ratio is increased, the FAME yield in the biodiesel product increases considerably. When a packed-bed catalytic reactor configuration is used, maximum yield is obtained when the methanol/oil molar ratio reaches 24:1 value, viscosity (4.4 cSt) and methyl esters content (97.1 %) values in the biodiesel reaction product fit with those values required by the standard UNE-EN 14124. Therefore, it has been demonstrated that methanol feed in excess is necessary.
The same behavior was observed when studying the reaction in a slurry reactor configuration. High reaction conversion was obtained when the methanol/oil molar ratio was increased. When the methanol/oil molar ratio was 24:1, the maximum reaction yield (96.0 %) was obtained. As can be seen, the same FAME yields were obtained for both catalytic reactor configurations using the same operation conditions.
The effect of packed-bed height on FAME yield of the obtained biodiesel product was examined. According to the results shown in Fig. 3, maximum conversions were obtained for packed-bed lengths higher than 8 cm, yet additional catalytic bed length made only few improvements in FAME yield in the biodiesel obtained.
Reaction time effect on the FAME yield of biodiesel obtained was studied, varying reaction time in a range from 15 min to 2 h. As can be seen in Fig. 4, the FAME yield in biodiesel reaction product increased with the reaction time until a FAME yield of 98.0 % was reached after 0.5 h reaction time when a slurry reactor configuration was used. The same behavior could be observed when a packed-bed reactor was used, but reaction times higher than those used in a slurry reactor were needed to obtain equilibrium conversions. This might be due to the fact that no material transfer limitations were occurring in the slurry reactor. On the other hand, though high reaction times were needed, packed-bed reactor configuration showed great advantages from a product separation point of view. In the slurry reactor configuration operation, remains of solid catalyst were found together with reaction products; however, using the packed-bed reactor operation, no catalyst damage evidences were observed and sudden separation into two phases from product emulsion was achieved.
The influence of the reaction temperature on the heterogeneously catalyzed transesterification reaction was examined by evaluating a temperature range from 50 to 60 °C. The reaction conversion increased slightly when temperature was increased from 50 to 60 °C. However, using 55 °C reaction temperature, 2 h reaction time, 20:1 methanol/oil molar ratio, and 8.2 cm catalytic packed-bed length, the value of the content in FAME of the biodiesel required by the standard UNE-EN 14124 (96.5 %) was achieved, reaching a 99.5 % value. The same results were found for transesterification reaction in a slurry reactor configuration.
In this work, the transesterification reaction using waste frying oil as feedstock for biodiesel production was also studied. Optimum reaction conditions obtained from the study of the sunflower oil transesterification reaction (Table 2) were used as a basis, obtaining a 51.3 % FAME yield from waste oil transesterification reaction. Methanol/oil molar ratio was then increased in order to improve the reaction conversion because it is one of the most important variables affecting FAME yield. When the packed-bed reactor configuration was used, FAME yield increased as the molar ratio increased, achieving the highest FAME yield (95.9 %) when a 28:1 methanol/oil molar ratio was used. The same trend was found when a slurry reactor configuration was used for waste oil transesterification reaction; the FAME yield remained practically constant for methanol/oil molar ratios >24:1 (Fig. 5).
We have demonstrated the possibility to use a packed-bed reactor configuration for biodiesel production, showing some important advantages: easy product separation and catalyst particles mechanical stability were achieved. Moreover, the presented results show that the same behavior was exhibited both in the slurry batch reactor system and in the packed-bed catalytic reactor system, reaching similar reaction rates.
Moreover, Table 1 shows the parameters as biofuel of the biodiesel product obtained under these optimum conditions. As can be seen, K-Pumice was able to simultaneously catalyze esterification and transesterification reactions for biodiesel synthesis, even when a waste vegetable oil with large amounts of FFA was used as feedstock. High yield of biodiesel were attained without producing soaps.
Potassium measurements have been conducted in biodiesel samples by atomic absorption spectrometry technique (Perkin-Elmer AAnalyst 300, UNE-EN 14108) in order to investigate catalyst leaching into the biodiesel. Results have been provided in Table 1. As can be seen, potassium concentration in biodiesel is very low and it can be minimized when the catalyst is used in a packed-bed reactor configuration related to its use in slurry reactor configuration. When waste oil is used as feedstock, potassium concentration in biodiesel slightly increase coming from the frying process to which the feedstock oil has been previously submitted. The process of food frying contributes to the increase of the content of potassium in waste oil by concentration or by transfer from the food composition.
In order to study the degree of leaching of the potassium ions from the heterogeneous catalyst, methanol recovered from one heterogeneously catalyzed reaction was mixed with sunflower oil. The mixture was maintained at the usual reaction operation conditions in order to evaluate the transesterification reaction conversion. No conversion was observed, indicating that the catalysis process was not homogeneous catalysis caused by leaching of potassium ions into methanolic phase.
In order to test the reusability of the K-Pumice, several reuses were carried out using sunflower oil as feedstock. The solid catalyst was reused after each run without any washing treatment. Figure 6 shows the FAME yield obtained versus the run number. A remarkable reduction in catalytic activity was observed, yielding 99.5 % of FAME in the first run and 35.8 % in the second run. FAME yield continued to decline until 4.0 % for the fifth run. This decay could be due to the deactivation of active sites due to their poisoning by some molecules present in the reaction mixture or due to a leaching of the catalytic active phase to the glycerin phase [20, 21]. Martín Alonso et al. [22] found a similar behavior when potassium was supported on alumina.
The K-Pumice reactivation process showed to be a solution to these activity decay problem [16]. This stable material (K-Pumice) could be used in a catalytic packed-bed configuration reactor and a reactivation reaction system could be developed in order to obtain continuous biodiesel production without any inconvenient decrease in catalytic activity. The catalyst reactivation process might be based on the same support material, only a cheap K-interchanging process of the same material should be developed.
Conclusions
Low loadings of potassium on natural pumice material lead to the formation of basic sites on the natural acid support. This bifunctional catalyst was able to catalyze simultaneously the esterification of FFA and transesterification of triglycerides molecules present in waste cooking oils.
K-Pumice catalyst activity showed in a catalytic packed-bed reactor system was essentially equal to the activity obtained using a slurry reactor system. No significant reaction rate differences were observed when a catalytic packed-bed reactor was used in order to minimize the mechanical damage of the catalyst due to continuous stirring produced by the slurry reactor operation.
K-Pumice, used as catalyst in a packed-bed reactor configuration, is a useful system to obtain biodiesel from renewable feedstock as waste oils. Advantages from a product separation point of view were observed.
The catalytic reactor system studied clearly shows advantages in the products separation and in the simple operation. It can be used as a basis to scale up a continuous biodiesel production system.
The biodiesel production method studied shows to be an environmentally benign and economical process with potentially wide applications in an industrial-scale production of biodiesel from low-quality oils containing high FFA and water contents.
References
Yu X, Wen Z, Lin Y, Tu S, Wang Z, Yan J (2010) Intensification of biodiesel synthesis using metal foam reactor. Fuel 89:3450–3456
Georgogianni KG, Katsoulidis AK, Pomonis PJ, Manos G, Kontominas MG (2009) Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process Technol 90:1016–1022
Parlak A, Karabas H, Ayhan V, Yasar H, Soyhan HS, Ozsert I (2009) Comparison of the variables affecting the yield of tobacco seed oil methyl ester for KOH and NaOH catalysts. Energy Fuel 23:1818–1824
Dias JM, Alvim-Ferraz MCM, Almeida MF (2008) Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel 87:3572–3578
Hsieh L, Kumar U, Wu JCS (2010) Continuous production of biodiesel in a packed-bed reactor using shell-core structural Ca(C3H7O3)2/CaCO3 catalyst. Chem Eng J 158:250–256
Kusdiana D, Saka S (2004) Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour Technol 91:289–295
Xie WL, Peng H, Chen LG (2006) Calcined Mg-Al hydrotalcites as solid base catalyst for methanolysis of soybean oil. J Mol Catal A Chem 246:24–32
Xie W, Yang Z (2007) Ba-ZnO catalyst for soybean oil transesterification. Catal Lett 117:159–165
Neri G, Rizzo G, De Luca L, Corigliano F, Arrigo I, Aricò AS et al (2008) Pt catalysts supported on zeolitized-pumice for the selective hydrogenation of campholenic aldehyde: a characterization and kinetic study. Appl Catal Gen 350:169–177
Álvarez-Galván MC, Brito A, García-Álvarez FJ, De la Peña O’Shea VA, Borges ME, Pawelec B (2008) Catalytic behaviour of bifunctional pumice-supported and zeolite/pumice hybrid catalysts for n-pentane hydroisomerization. Appl Catal Gen 350:38–45
Chuan XY, Hirano M, Inagaki M (2004) Preparation and photocatalytic performance of anatase-mounted natural porous silica, pumice, by hydrolysis under hydrothermal conditions. Appl Catal B Environ 51:255–260
Gök A, Göde F, Türkaslan BE (2006) Synthesis and characterization of polyaniline/pumice (PAn/Pmc) composite. Mater Sci Eng B 133:20–25
Gelbard G (2005) Organic synthesis by catalysis with ion-exchange resins. Ind Eng Chem Res 44:8468–8498
Kiss M, Losonczi B, Morgos J, Rusznak I, Haklits I (1980) Study of alkylating reactions catalyzed by cation-exchange resins. J Chromatogr A 201:383–389
Ni J, Meunier FC (2007) Esterification of free fatty acids in sunflower oil over solid acid catalyst using bath and packed bed-reactors. Appl Catal Gen 333:122–130
Borges ME, Díaz L, Alvarez-Galván MC, Brito A (2011) High performance heterogeneous catalyst for biodiesel production from vegetal and waste oil at low temperature. Appl Catal B Environ 102:310–315
Shibasaki-Kitakawa N, Honda H, Kuribayashi H, Toda T, Fukumura T, Yonemoto T (2007) Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst. Bioresour Technol 98:416–421
Gelbard G, Brès O, Vargas RM, Vielfaure F, Schuchardt UF (1995) 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. J Am Oil Chem Soc 72:1239–1241
Borges ME, Díaz L, Gavín J, Brito A (2011) Estimation of the content of fatty acid methyl esters (FAME) in biodiesel samples from dynamic viscosity measurements. Fuel Process Technol 92:597–599
Liu H, Su L, Liu F, Li C, Solomon U (2011) Cinder supported K2CO3 as catalyst for biodiesel production. Appl Catal B Environ 106:550–558
Chiu C, Goff M, Suppes G (2005) Distribution of methanol and catalysts between biodiesel and glycerin phases. AICHE J 51(4):1274–1278
Martín Alonso D, Mariscal R, Moreno-Tost R, Zafra Poves MD, López Granados M (2007) Potassium leaching during triglyceride transesterification using K/γ-Al2O3 catalysts. Catal Commun 8:2074–2080
Acknowledgments
The authors acknowledge a research grant support by Programa de FPU del Ministerio de Educación, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borges, M.E., Díaz, L. Catalytic Packed-Bed Reactor Configuration for Biodiesel Production Using Waste Oil as Feedstock. Bioenerg. Res. 6, 222–228 (2013). https://doi.org/10.1007/s12155-012-9246-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9246-7