Abstract
The increased emphasis on research of dedicated biomass and biofuel crops begs for biotechnology method improvements. For switchgrass (Panicum virgatum L.), one limitation is inefficient tissue culture and transformation systems. The objectives of this study were to investigate the utility of a new medium described here, LP9, for the production and maintenance of switchgrass callus and its regeneration, which also enables genetic transformation. LP9 medium is not based on Murashige and Skoog (MS) medium, the basal medium that all published switchgrass transformation has been performed. We demonstrate an efficient tissue culture system for switchgrass Alamo 2, which yields increased viability of callus and the ability to maintain callus for a duration of over 6 months. This longevity gives a greater useful callus lifetime than for published switchgrass MS-based media. This increased longevity enables greater potential efficiency and throughput for a transformation pipeline. Callus produced on LP9 is categorized as type II callus, which is more friable and easier to multiply, maintain and transfer than type I callus obtained from previously described tissue culture systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing interest in the production of biofuels has warranted research in the production and genetic manipulation of high biomass crops such as switchgrass (Panicum virgatum L.), a warm-season perennial grass native to North America. With this increased interest, it is necessary to develop higher throughput transformation systems that are enabled by an efficient and reliable tissue culture system for target tissue production and plant regeneration. Stable transformation, in turn, enables the reverse genetics research for cell wall manipulation and plant growth improvement. Current switchgrass tissue culture and transformation systems are not very efficient and limited to derivatives of a single variety: Alamo. There are currently two described tissue culture systems in switchgrass: embryogenic callus [1–5] and seed-derived callus [6, 7]. Limitations of these systems include longevity of embryo viability (typically, less than 2 months) and high genetic and response variability in the seed-derived system. Despite the increased interest in switchgrass tissue culture, there has been little recent progress to enhance switchgrass tissue culture systems.
Callus in grasses can be classified as type I or type II, based upon color, texture, regeneration system, and the amount of time required for callus initiation. The morphology of callus has been reported and described in the important agronomic monocot crops such as maize [8–14], rice [15–17], sorghum [18], sugarcane [19], wheat [20], and various nonfood grasses [21–26]. Type I callus is the typical and most prevalent callus formed in monocot species. It is characterized by compact form, slow-growth, white to light yellow in color, and highly organized [27]. This callus is composed almost entirely of cytoplasmic meristematic cells that lack large vacuoles. In maize, type I callus can only be maintained for only a few months and cannot be used in suspension cultures; whereas, type II callus can be maintained in culture for extended periods of time and is able to form cell suspensions [8, 27, 28]. Type II callus derived from maize has been described as soft, friable, rapidly growing, and exceedingly regenerative but is typically formed at lower frequencies than type I callus [8, 27–29]. Switchgrass callus described to date has all been of type I. In this study, we describe a novel non-Murashige and Skoog (MS)-based media and culture conditions that result in the production of type II callus in switchgrass that can be useful for the production of transgenic plants (Figs. 1 and 2).
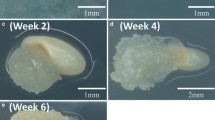
Nontransgenic and transgenic switchgrass transplanted into bark media in pots. The dissecting micrographs were produced using the lowest power of an epifluorescence dissecting microscope. a Switchgrass transformed with pporRFP and c nontransgenic under 3 ms white light exposure. b Switchgrass transformed with pporRFP and d nontransgenic excited with 535/30 nm light and emission filter 600/50 nm exposed for 15 s. e Nontransgenic roots of switchgrass and g switchgrass transformed with pporRFP under white light and f nontransgenic excited and h switchgrass transformed with pporRFP excited with 535/30 nm light and emissions filter 600/50 nm exposed for 15 s
Methods
Callus Induction, Maintenance, and Plant Regeneration
Switchgrass Alamo 2 tillers grown in the greenhouse were excised from plants at the E2 to E4 stage [30]. Inflorescences were cut 7 mm above the fourth node [1]. Pieces of inflorescence were then sterilized for 35 min in 75% commercial bleach containing 1% Tween 20. Inflorescences were subsequently washed with sterile water three times. Ends were then removed and discarded, and inflorescences were cut longitudinally in half, placed on MS + benzyladenine (BA), and incubated at 25°C in the dark as performed previously [1]. It is at this point in our protocol that we have diverged from previous tissue culture methodologies of embryogenic callus production [1] to produce friable type II callus.
After 10 days on MS + BA, inflorescences were removed from the media and placed into a sterile Petri dish, further cut into 1-cm-long segments and placed onto LP9, a new callus induction media modified from the callus induction medium by Lu et al. [31]. This media by Lu et al. [31] was altered by adding the auxin 2,4-dichlorophenoxyacetic acid (2,4-D; 5 mg l−1) in place of dicamba, removing BA and myo-inositol from the media, and decreasing the amount of proline from 500 to 100 mg l−1. These determinations were made after informal experimentation with switchgrass (data not shown). After 14 days on LP9, callus developed (Fig. 3b). Callus was then excised from the explants and cultured further on fresh LP9.
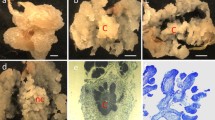
Two tissue culture media were used to produce callus from Alamo 2 inflorescence. a Callus derived from the methods of [1] and b callus derived from LP9 media. Type II callus was observed on c LP9, and type I callus was observed on d MSO + 2,4-D + BAP media [1] in switchgrass. Alamo 2 callus grown on LP9 for e 1 and f 6 months. Formation of g Alamo 2 shoots at 2 weeks and h rooted Alamo 2 shoot
Antibiotic Selection
Callus production under hygromycin selection was performed to determine optimal selection rates that can be used for genetic transformation. Four concentrations of hygromycin B (0, 50, 60, and 70 mg l−1; Calbiochem, Gibbstown, NJ, USA) in LP9 (5 mg l−1 2,4-D, 100 mg proline, N6 macroelements, B5 microelements, Fe-EDTA, supplemented with Gamborg’s vitamins, 500 mg l−1 casein hydrolysates, 500 mg l−1 glutamine, 30 g l−1 sucrose, 3 g l−1 Gelzan) [31] were used to determine kill curves for six replicated plates for each treatment containing 20 explants per plate. Growth was assessed by weighing fresh callus pieces at days 0 and 60. Subculturing was performed at 3-week intervals. Data were analyzed by analysis of variance (ANOVA) using the general linear model (SAS 9.2, Cary, NC, USA). Duncan’s multiple range test was used to compare treatment mean values when significant differences (at the 0.05 probability level) were found.
Vector Construction
The binary vector contained a red fluorescent protein (pporRFP gene from the coral Porites porites) gene under the control of the maize ubiquitin (ZmUbi1) promoter and the selectable marker gene encoding hygromycin phosphotransferase (hph) that was under the control of the rice actin 1 (OsAct1) promoter. The ZmUbi1 promoter from the pAHC25 plasmid [32] was polymerase chain reaction (PCR) amplified and cloned into pCR4B-TOPO (Invitrogen, Carlsbad, CA, USA). The pEarleyGate 304 plasmid contains the Gateway compatible cassette attR1-CmR-ccdB-attR2 coupled with an AcV5 epitope and the OCS terminator [33]. This cassette was PCR amplified and cloned directly downstream of the ZmUbi1 promoter in pCR4B-TOPO. To confer resistance to hygromycin, a cassette containing the OsAct1 promoter and hph gene was cloned upstream of the ZmUbi1- attR1-CmR-ccdB-attR2 cassette in pCR4B-TOPO. The OsAct1-hph and ZmUbi1- attR1-CmR-ccdB-attR2 cassettes were excised with SbfI and AscI and gel purified with QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). The pPZP201BK binary backbone [34] was digested with PstI and AscI, purified with the QIAquick PCR Purification Kit (Qiagen), and ligated with the OsAct1-hph and ZmUbi1- attR1-CmR-ccdB-attR2 cassettes. The pporRFP gene [35] was kindly provided by Dr. Mikhail Matz and was cloned into pCR8/GW/TOPO and recombined into the expression vector using Gateway® LR Clonase® II enzyme mix (Invitrogen). All amplified regions and resulting plasmids were sequence verified at the University of Tennessee Molecular Biology Resource Facility.
Agrobacterium-Mediated Transformation
Transformation was conducted using Agrobacterium tumefaciens strain EHA105 containing the vector described above. Agrobacterium was grown in YEP supplemented with 50 mg l−1 kanamycin at 27°C for 2 days. Cultures were centrifuged at 4,000 rpm for 30 min. Supernatant was removed, and Agrobacterium was resuspended in 25 ml of liquid LP9, 100 μM of acetosyringone, and 10 μl of Silwet. Agrobacterium solution was then shaken at 150 rpm at room temperature for 30 min. Callus pieces, 0.5 cm, were placed in the Agrobacterium solution and incubated at room temperature under 0.53 atm vacuum for 30 min. Callus pieces were then coincubated on LP9 for 3 days. Callus pieces were placed in liquid LP9 + 400 mg l−1 timetin and vortexed to remove excess Agrobacterium growth. Callus pieces were placed on LP9 + 60 mg l−1 hygromycin + 400 mg l−1 timetin. Calli were transferred to new LP9 media + antibiotics every 2 weeks until transgenic callus formed and grew to 0.5 cm. Calli were then placed on MSO + 5 μM BAP + 60 mg l−1 hygromycin + 400 mg l−1 timetin. Shoots were formed after 14 days and were placed onto MS as described above to regenerate plants.
Histology
Scanning electron microscopy (SEM) was performed whereby tissues (5 weeks) were fixed in 3% (v/v) glutaraldehyde and 0.1 M cacodylate buffer. Samples were then rinsed three times (10 min each) in cacodylate buffer and subsequently postfixed in cacodylate-buffered 2% osmium tetroxide for 90 min and subsequently dehydrated in acetone in series (25%, 50%, 75%, 90%, 100%, and dry 100%). Following acetone dehydration, the samples were critical point dried with liquid carbon dioxide (Ladd Research Industries Critical Point Dryer). Dried samples were stored under vacuum until viewing. Five calli were affixed to two-sided carbon tape on a stub and sputtered with gold (SPI Sputter coater) prior to viewing with SEM. Samples were observed with a LEO (Zeiss) 1525 FE-SEM.
Results
Callus Induction, Maintenance, and Plant Regeneration
LP9 medium produced stable type II friable callus (Fig. 3b) for switchgrass Alamo 2 that is morphologically and functionally unique compared with that from previous research (Fig. 3a, d) [1]. Callus produced from this media is brittle and white (Fig. 3b, c), which allowed for easy multiplication and transfer, and less “artistic judgment” to select callus that will subsequently proliferate. This morphology is consistent with descriptions of type II callus previously shown in maize [8, 10, 13]. Callus forms quickly, after 2 weeks, on LP9 media from inflorescent explants on 100% of inflorescent explants, which is similar to that achieved from the published methods [1]. Callus can easily be removed from the cut explants and placed onto fresh media, forming type II callus, which proliferated at an efficiency of 33%; i.e., approximately 67% of the callus is type I. Once formed, type II callus was transferred every 3 weeks until it was used for transformation experiments or plant regeneration. Callus induced from LP9 media has demonstrated longevity in our laboratory, thus far, for over 6 months compared with embryogenic callus derived from MS + 2,4-D + 6-benzylaminopurine (BAP) that tends to become unresponsive after 3–4 months (Fig. 3e, f) ( [1]; data not shown). In practice, since callus can be divided and utilized for longer than 6 months, a genetic transformation pipeline is potentially much more efficient and faster since new explants are not needed as frequently (Fig. 3f). After 1 month on LP9, callus can be selected and used for A. tumefaciens-mediated transformation or particle bombardment, and plants begin to be regenerated within 3 weeks of callus initiation.
Shoots were readily produced in 2 weeks after placement onto MS medium containing 5 μM BAP [1] (Fig. 3g). Once shoots were produced, individual shoots were separated from the clump and placed into MS in Magenta GA7 boxes, and rooting occurred in about 2 weeks (Fig. 3h).
Transformation
Five independent transgenic events originating from separate callus pieces were obtained using this method from 113 callus clusters exposed to Agrobacterium, i.e., 4.4% transformation efficiency. However, these 113 callus clusters consisted of type I and type II (approximately half of each), but only type II callus yielded transgenic plants; therefore, this transformation efficiency can likely be increased by selecting only type II callus for transformation experiments. These results are comparable to published efficiency from prior methods. Somleva et al. [36] confirmed that 27 transgenic events were recovered from 794 callus clusters exposed to Agrobacterium, i.e., 3.4% efficiency. Entire plant expression of pporRFP was visualized using the lowest power of an epifluorescence dissecting microscope excited with 535/30 nm light and emissions filter 600/50 nm exposed for 15 s (Figs. 1 and 2). PporRFP has an excitation maximum of 578 nm and an excitation maximum of 595 nm [35] and gives bright fluorescence in transgenic plants (Figs. 1 and 2).
Antibiotic Selection
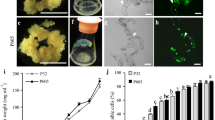
After 2 months on selection, fresh weights were found to be significantly different in hygromycin treatments (P < 0.05; Fig. 4). Fresh weights obtained from calli grown on selection with 60 mg l−1 hygromycin was not significantly different from those grown on 70 mg l−1 hygromycin selection (P < 0.05), but these were much less than those grown on 50 mg l−1 hygromycin (Fig. 4). Thus, the apparent optimal concentration for subsequent selection of transgenic calli was determined to be 60 mg l−1 hygromycin.
Fresh weights (grams) of callus grown under hygromycin selection. Six plates of callus were grown in LP9 with each of four concentrations of hygromycin (0, 50, 60, or 70 mg l−1). Fresh weights were recorded on day 0 and 60. Data were analyzed by ANOVA using the general linear model (SAS 9.2, Cary, NC, USA). Duncan’s multiple range test was used to compare treatment mean values within time points for significant differences (at the 0.05 probability level). Error bars indicate standard error. Different letters at each time point indicates significant differences
Histology
A scanning electron micrograph of callus produced on LP9 (Fig. 5) shows clusters of somatic embryos that are found as protrusions along the leftmost surface. Various morphologies were observed in this type II callus produced on LP9 (Fig. 5). These morphologies include irregular surfaces with globular, unorganized tissue types. Tissues of this type have demonstrated to have rapid growth and be highly friable.
Discussion
Recent progress to improve switchgrass tissue culture systems and transformation has been limited. Previous tissue culture systems for switchgrass have manipulated various auxins and cytokinins in an MS-based system [3–6]. Plant regeneration by both somatic embryogenesis and organogenesis occurred from both mature caryopses and young leaf segments of switchgrass Alamo at 45 and 5 μM BAP, respectively, in combination with 22.5 μM 2,4-D [3]. Denchev and Conger [4] examined the influence of type and concentration of two auxins, 2,4-D and picloram, in combination with the cytokinin, BA, on callus induction and regeneration in switchgrass Alamo mature caryopses and determined that 11.3–45.0 μM 2,4-D in combination with 15.0 or 45.0 μM BA produced optimal results. Various combinations of 2,4-D and another cytokinin, thidiazuron (TDZ), have also been researched for their ability to produce a highly regenerative tissue culture system in switchgrass cv. Alamo [6]. The best combination was determined to be 4.5 μM 2,4-D and 18.2 μM TDZ [6]. Dutta Gupta and Conger [5] were able to establish embryogenic cell suspension cultures of switchgrass when MS media were supplemented with 9.0 μM 2,4-D and 4.4 μM BAP. However, tissue culture systems using MS in combination with 2,4-D and various cytokinins produced a callus that was hard, white, and compact at the coleptilar or scutellar stage of embryogenesis and, therefore, is classified as type I callus [3, 5, 37].
LP9 is not an MS-based tissue culture medium, but rather combines N6 macroelements and B5 microelements with the auxin, 2,4-D. It does not include any cytokinin. This system is novel and enables callus production and plant regeneration in switchgrass. Lu et al. [31] utilized media with similar components for bermuda grass tissue culture, but their protocol did not result in friable callus with type II characteristics. However, we have demonstrated that LP9 produces type II switchgrass callus that is highly friable and has increased longevity compared with prior media. Most maize tissue culture systems that produce type II callus demonstrating friability and increased longevity utilize N6 macroelements as their media base [8, 10, 13]. However, we are the first to examine the callus induction and plant regeneration using N6 macroelements combined with B5 microelements in switchgrass tissue culture.
Armstrong and Green [8] found that the addition of L-proline to N6 media produced a friable, type II callus from immature maize embryos and further concluded that proline might function to protect the cultures from various stresses. Interestingly, the addition of l-proline to MS media did not enhance formation of somatic embryos in maize [8].
LP9 supplemented with hygromycin (60 mg l−1) can be used to select for transformants similar to those selected using bialaphos (10 mg l−1) in an MS-based system [38, 39].
Here, we have demonstrated an improved tissue culture system for switchgrass Alamo 2 that features a friable, fast-growing callus derived from inflorescences that can be classified as type II callus using our new media, LP9. Callus produced on LP9 demonstrates increased viability and can be maintained for longer periods of time (greater than 6 months), enabling use in transformation experiments without having to regenerate callus from new tillers. This enhanced maintenance and regeneration reduces the time needed to produce whole transgenic plants by at least 1 month. In addition, type II callus is easily recognized by its friable and dry appearance and lighter color, which can be chosen without the aid of a microscope for subsequent proliferation. Further work needs to be done to compare transformation efficiencies of switchgrass callus produced on LP9 to those produced on media in the current literature as well as examine genotype specificity.
Abbreviations
- BA:
-
Benzyladenine
- BAP:
-
6-Benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- SEM:
-
Scanning electron microscopy
- TDZ:
-
Thidiazuron
References
Alexandrova KS, Denchev PD, Conger BV (1996) In vitro development of inflorescences from switchgrass nodal segments. Crop Sci 36:175–178
Alexandrova KS, Denchev PD, Conger BV (1996) Micropropagation of switchgrass by node culture. Crop Sci 36:1709–1711
Denchev PD, Conger BV (1994) Plant regeneration from callus cultures of switchgrass. Crop Sci 34:1623–1627
Denchev PD, Conger BV (1995) In vitro culture of switchgrass: influence of 2,4-D and picloram in combination with benzyladenine on callus initiation and regeneration. Plant Cell Tissue Organ Cult 40:43–48
Dutta Gupta S, Conger BV (1999) Somatic embryogenesis and plant regeneration from suspension cultures of switchgrass. Crop Sci 39:243–247
Dutta Gupta S, Conger BV (1998) In vitro differentiation of multiple shoot clumps from intact seedlings of switchgrass. In Vitro Cell Dev Biol-Plant 34:196–202
Seo M-S, Takahara M, Ebina M, Takamizo T (2008) Evaluation of tissue culture response from mature seeds of Panicum spp. Grassland Sci 54(3):125–130
Armstrong CI, Green CE (1985) Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 164:207–214
Assam SK (2001) Callus production and plant regeneration in Egyptian maize genotypes. Arab J Biotechnol 4:247–256
Frame BR, Zhang H, Cocciolone SM, Sidorenko LV, Dietrich CR, Pegg SE et al (2000) Production of transgenic maize from bombarded type II callus: effect of gold particle size and callus morphology on transformation efficiency. In Vitro Cell Dev Biol-Plant 36:21–29
Lu C, Vasil IK, Ozias-Akins P (1982) Somatic embryogenesis in Zea mays L. Theor Appl Genet 62:109–112
McCain JW, Kamo KK, Hodges TK (1988) Characterization of somatic embryo development and plant regeneration from friable maize callus cultures. Bot Gazette 149:16–20
Songstad DD, Petersen WL, Armstrong CL (1992) Establishment of friable embryogenic (type II) callus from immature tassels of Zea mays (Poaceae). Am J Bot 79:761–764
Welter ME, Clayton DS, Miller MA, Petolino JF (1995) Morphotypes of friable embryogenic maize callus. Plant Cell Rep 14:725–729
Chen T-S, Lam L, Chen S-C (1985) Somatic embryogenesis and plant regeneration from cultured young inflorescences of Oryza sativa L. (rice). Plant Cell Tissue Organ Cult 4:51–54
Nakamura T, Maeda E (1989) A scanning electron microscope study on Japonica type rice callus cultures, with emphasis on plantlet initiation. Japan J Crop Sci 58:395–403
Rueb S, Leneman R, Schilperoort RA, Hesngens LAM (1994) Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.). Plant Cell Tissue Organ Cult 36:259–264
Jeoung JM, Krishnaveni S, Muthukrishnan S, Trick HN, Liang GH (2002) Optimization of sorghum transformation parameters using genes for green fluorescent protein and β-glucuronidase as visual markers. Hereditas 137:20–28
Guiderdoni E, Demarly Y (1988) Histology of somatic embryogenesis in cultured leaf segments of sugarcane plantlets. Plant Cell Tissue Organ Cult 14:71–88
Redway FA, Vasil V, Lu D, Vasil IK (1990) Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.). Theor Appl Genet 79:609–617
Bajaj YPS, Sidhu BS, Dubey VK (1981) Regeneration of genetically diverse plants from tissue cultures of forage grass—Panicum spp. Euphytica 30:135–140
Chaudhury A, Qu R (2000) Somatic embryogenesis and plant regeneration of turf-type bermudagrass: effects of 6-benzyladenine in callus induction medium. Plant Cell Tissue Organ Cult 60:113–120
Lu C, Vasil IK (1981) Somatic embryogenesis and plant regeneration from leaf tissue of Panicum maximum Jacq. Theor Appl Genet 59:275–280
Lu C-Y, Vasil IK (1981) Somatic embryogenesis and plant regeneration from freely-suspended cells and cell groups of Panicum maximum Jacq. Ann Bot 48:543–548
Lu C-Y, Vasil IK (1985) Histology of somatic embryogenesis in Panicum maximum (guinea grass). Amer J Bot 72:1908–1913
Zhang S, Hanna W, Ozias-Akins P (2007) Comparison of callus induction and plant regeneration from different explants in triploid and tetraploid turf-type bermudagrass. Plant Cell Tissue Organ Cult 90:71–78
Vasil IK, Vasil V (1994) In vitro cultures of cereals and grasses. In: Vasil IK, Thorpe TA (eds) Plant cell and tissue culture. Kluwer Academic Publishers, Dordrecht, p 293
Vasil V, Vasil IK (1986) Plant regeneration from friable embryogenic callus and cell suspension cultures of Zea mays L. J Plant Physiol 123:211–227
Vasil V, Vasil IK, Lu C (1984) Somatic embryogenesis in long-term callus cultures of Zea mays. Am J Bot 71:158–161
Moore KJ, Moser LE, Vogel KP, Waller SS, Johnson BE, Pedersen JF (1991) Describing and quantifying growth stages of perennial forage grasses. Agron J 83:1073–1077
Lu S, Wang Z, Peng X, Guo Z, Zhang G, Han L (2006) An efficient callus suspension culture system for triploid bermudagass (Cynodon transvaalensis × C. dactylon) and somaclonal variations. Plant Cell Tissue Organ Cult 87:77–84
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K et al (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45(4):616–629
Covert SF, Kapoor P, M-h L, Briley A, Nairn CJ (2001) Agrobacterium tumefaciens-mediated transformation of Fusarium circinatum. Mycol Res 105(3):259–264
Alieva NO, Konzen KA, Field SF, Meleshkevitch EA, Hunt ME, Beltran-Ramirez V et al (2008) Diversity and evolution of coral fluorescent proteins. PLoS ONE 3(7):e2680
Somleva MN, Snell KD, Beaulieu JJ, Peoples OP, Garrison BR, Patterson NA (2008) Production of polyhydroxybutyrate in switchgrass a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnol J 6:663–678
Shaeffer WI (1990) Terminology associated with cell, tissue and organ culture, molecular biology and molecular genetics. In Vitro Cell Dev Biol 26:97–101
Richards HA, Rudas VA, Sun V, McDaniel JK, Tomaszewski Z, Conger BV (2001) Construction of a GFP-BAR plasmid and its use for switchgrass transformation. Plant Cell Rep 20:48–54
Somleva MN, Tomaszewski Z, Conger BV (2002) Agrobacterium-mediated genetic transformation of switchgrass. Crop Sci 42:2080–2087
Acknowledgments
This work was funded by grants obtained from the Bioenergy Science Center. The BioEnergy Science Center is a US Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burris, J.N., Mann, D.G.J., Joyce, B.L. et al. An Improved Tissue Culture System for Embryogenic Callus Production and Plant Regeneration in Switchgrass (Panicum virgatum L.). Bioenerg. Res. 2, 267–274 (2009). https://doi.org/10.1007/s12155-009-9048-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-009-9048-8