Abstract
Shikimic acid properties and its available analytical techniques are discussed. Plants having the highest content of shikimic acid are shown. The existing isolation methods are analyzed and the most optimal approaches to extracting this acid from natural sources (plants and microorganisms) are considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shikimic acid (3,4,5-trihydroxy-1-cyclohexene-1-carboxylic acid), a natural organic compound, is an important intermediate in the biosynthesis of lignin [1], aromatic amino acids (phenylalanine, tyrosine, and triptophane), and most alkaloids of plants and microorganisms [2–4].
Shikimic acid is generally utilized as a starting material for industrial synthesis of the antiviral Oseltamivir (this drug against the H5N1 influenza virus is administered to treat and prevent all the known strains of influenza virus) [2, 5]. There are reports on the shikimic acid-based synthesis of (−)-zeylenone that is widely employed as a preparation for chemotherapy of cancerous diseases [6]. There are data available on the synthesis of monopalmityloxy shikimic acid possessing an anticoagulant activity and capable of reducing blood coagulability when injected intramuscularly [7]. A Chinese research team has synthesized a shikimic acid derivative, triacetylshikimic acid, which exhibits anticoagulant and antithrombotic activities [8].
In addition, shikimic acid derivatives represent a great interest for agriculture because many of them are used as herbicides and antibacterial agents inasmuch as they can block the shikimate pathway in plants and bacteria without a negative effect toward mammals [9, 10]. Shikimic acid also has the potential to be used for benzene-free production of phenol [11].
Thus, shikimic acid can be used and is being used as a reactant in organic synthesis in both basic science and applied fields of organic chemistry and medicine, particularly to obtain various medicinal drugs.
In the present review, we set a goal to show in a critical comparison as to which methods allow the production and isolation of this important raw material from natural sources.
Physicochemical properties of shikimic acid
Shikimic acid is a white crystalline substance with a melting point of 190 °C [12–14]. This compound is levorotary and has the specific rotation [α] = –157° × cm3/g × dm−1 [12, 14, 15]. The single maximum in the UV range is recorded at λ = 235 nm [12, 14, 16–18]. A great many isomers have been described for shikimic acid [14, 18–25], but only the α-isomer [26–29], whose structure is depicted in Fig. 1, has biological activity.
The shikimic acid reactions are characteristic of a compound containing a carboxyl group, three hydroxyl groups, and a double bond. The stereochemistry of the molecule is such that the intramolecular reaction between the carboxyl group and a single hydroxyl group is possible under appropriate conditions. Adjustment reactions of cis-hydroxyl groups are also observed.
Analytical techniques
Paper chromatography
The simplest technique used to study shikimic acid and its derivatives is paper chromatography. With this technique, Perkins and Aronof [30] identified and purified shikimic acid from impurities in extracts.
These authors used Whatman 3MM paper in their work (see Table 1).
Color reactions
Neither shikimic acid nor quinic acid is colored under visible light or glows under ultraviolet light. Reagents giving generally satisfactory and reproducible results are listed in Table 2. Zaprometov [31], for instance, used in his work the technique of color reactions only to detect shikimic and quinic acids.
Quantitative analysis techniques
Several quantitative analysis techniques for shikimic acid have been devised, each comprising iodic acid-assisted oxidation as an initial step. The glycol degradation product is then treated with a chromogenic reagent, and the concentration is quantified by colorimetric analysis.
The first technique is that of Yoshida and Hasegawa [32]. Samples were oxidized in acetate buffer (pH 4.7), and the oxidation product was treated with aniline, resulting subsequently in a polymethine pigment. Spectra were measured at 510 nm—the wavelength at which the optical density is proportional to a concentration ranging from 40 to 200 μg/ml of shikimic acid. The other widespread organic acids and quinic acid are not involved in these reactions. The presence of excess polyhydroxy compounds tends to exaggerate results due to the reaction with iodic acid. The chromatographic purification of the extract using an ion exchange resin ensures more concise results. Aromatic hydroxy acids give a brown color when reacted with iodic acid, which can be adjusted by a control test with water instead of aniline.
Gaitonde and Gordon [33] found that the saturated yellow color was progressing when sodium hydroxide or barium hydroxide was added to the reaction mixture as soon as shikimic acid was treated with iodic acid. The color can be stabilized by adding glycine. The optical density was determined at λ = 380 nm for 10 min after the addition of the base. The absorption intensity begins to decrease when the solution ages. The lowest limit for this technique was about 0.3 μg/ml of shikimic acid.
Quinic acid exhibits a pale yellow color when reacted with sodium hydroxide, but the adsorption does not reach its maximum for about 20 min. Analyses of shikimic acid should be carried out once the base is added in order for quinic acid not to interfere. Among the other compounds that interfere in the reaction are gallic acid (3% of the optical density of shikimic acid), triptophane (2%), adrenaline (4%), NAD (2%), HADP, and TDF (6%).
The literature reports two procedures that make use of thiobarbituric acid as a chromogenic reagent. The first procedure belongs to Saslaw and Waravdekar [34]. Shikimic acid was oxidized by iodic acid in the presence of sulfuric acid. The excess iodic acid was removed by sodium arsenite, and thiobarbituric acid was added. The saturation of the blue solution, while taking off data at λ = 660, should be determined immediately once the color begins to become dull under prolonged exposure to light.
The sensitivity of this analysis is good; the linear reaction was observed from 1.0 to 12.0 μg/ml of shikimic acid. The reproducibility was 5%. Quinic acid yielded up to 3% of the absorbance observed for shikimic acid.
The second analysis procedure for shikimic acid using thiobarbituric acid was described by Millican [35]. Shikimic acid was oxidized by iodic acid using phosphoric acid as a reaction solvent. The excess iodic acid was removed by sodium arsenite followed by the addition of phosphoric acid. The reaction products have peaks at λ = 450, 535, and 660 nm. The red pigment was extracted into cyclohexanone and measured at λ = 535 nm. The chromogen absorption was linear at a concentration of shikimic acid between 0.01 and 0.06 μmol.
There was observed interference between deoxyribose derivatives and some shikimic acid derivatives. For instance, adenosine gave a 300% color yield based on shikimic acid; deoxyribose afforded a 200% color yield. The color yields of 5-dehydroshikimic acid, quinic acid, and 5-dehydroquinic acid were 60%, 18%, and 10%, respectively. 5-Phosphoshikimic acid provided no color under the aforesaid conditions.
NMR spectroscopy of shikimic acid
Shikimic acid is readily identified by NMR spectroscopy using such solvents as D2O, tetradeuteriomethanol, and acetone-d6. Shikimic acid has the following spectral characteristics: 1H NMR (300 MHz, D2O, δ): 6.70 (m, 1H), 4.30 (m, 1H), 3.93 (m, 1H), 3.67 (dd, J = 8.4, 4.5 Hz, 1H), 2.64 (dd, J = 18.0, 4.8 Hz, 1H), 2.12 (dd, J = 18.0, 6.3 Hz, 1H); 13C NMR (75 MHz, D2O, δ): 170.1, 137.1, 129.8, 75.1, 66.5, 65.8, 30.4 [3, 14, 32–38].
Reversed-phase HPLC of shikimic acid
Organic acids are identified under conditions of reversed-phase high-performance liquid chromatography (RP-HPLC). The elution order of organic acids under RP-HPLC conditions is as follows: tartaric acid < quinic acid < succinic acid < hydroxycitric acid < malic acid < isocitric acid < shikimic acid < ascorbic acid < fumaric acid < citric acid [39].
Other analytical techniques for shikimic acid
There exist a lot of reported methods to analyze organic acids, including shikimic acid. Ion exchange chromatography was employed to detect shikimic acid in wine and other objects [11, 41, 42]. High-performance liquid chromatography on a C18 reversed-phase column was used by Dainiak et al. [43] and García Romero et al. [44]. Tusseau and Benoit [45] employed a sulfonyl–styrene–divinylbenzene column. A NH2 column was used by Gibson et al. [11] and capillary zone electrophoresis by Mardones et al. [46]. However, due to the low ultraviolet optical density of shikimic acid, these methods require producing derivatives. In this connection, other analytical methods were suggested, which involve, for instance, gas chromatography (GC) [47] and gas chromatography–mass spectrometry (GC-MS) [48–50]. GC-MS with selected ion monitoring (SIM) became one of the most effective methods for the quantitative analysis of plants’ compositions [51, 52]. Ibarz et al. [53] reported the identification of carboxylic acids, including shikimic acid, in grapes using GC/MS-SIM [51, 52].
Shikimic acid in living organisms
The shikimate pathway
Shikimic acid was first isolated in Japan, 1885, from Illicium religiosum and was named after the Japanese name for this plant, shikimi-no-ki [53]. Only after 50 years was the complete molecular structure of this quite an interesting compound determined. In the early 1950s, a series of experiments showed that shikimic acid plays a key role in the formation of aromatic amino acids.
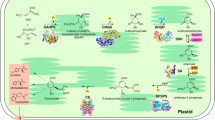
The shikimate pathway or pathway of cyclohexenecarboxylic acid displayed in Fig. 2 is widely distributed in microorganisms and plants where shikimic acid is a precursor for the biosynthesis of primary metabolites such as aromatic amino acids and folic acid and a great many other aromatic compounds. Since the basic element of all aromatic compounds—benzene ring—is formed in plants and microorganisms through the shikimate pathway, shikimic acid is an extremely essential compound in plants and microbes [54–57]. Only a limited number of plant phenols have aromatic rings that are synthesized through another mechanism, that is, the polyketide condensation of acetate units [58].
We believe that discussion of shikimic acid as an important compound would be incomplete without a more or less detailed examination of the shikimate pathway.
The initial components which form an aromatic ring through the shikimate pathway (Fig. 2) are phosphoenolpyruvate (1) resulting from glycolytic degradation of glucose and erythrose-4-phosphate (2), which is an intermediate product of the glucose oxidation through the pentose phosphate pathway. Their condensation leads to a heptacarbon compound, deoxy-d-arabino-heptulosonate 7-phosphate (3), which then goes through cyclization to give 3-dehydroquinic acid (4). The next step is that 3-dehydroquinic acid loses water and is transformed into 3-dehydroshikimic acid (5). Due to the oxidoreductase enzyme, 5 is further converted into shikimic acid (6), which is one of the most important intermediates of the pathway and, thanks to which, the latter has gained its name.
Shikimic acid is structurally similar to aromatic compounds; however, its six-membered carbon ring contains only one double bond. Further transformations of this ring begin with the phosphorylation of shikimic acid at the third carbon atom (7), and then the phosphoenolpyruvate molecule associates with the phospholyrated acid to form 5-enolpyruvilshikimate-3-phosphate (8). The latter subsequently undergoes dephosphorylation and dehydration, resulting in chorismic acid (9), which is another important intermediate whose ring contains already two double bonds.
At this point, the shikimate pathway divides. The first direction gives rise to l-tryptophan (and further to indolic derivatives) from chorismic acid; the second one forms l-phenylalanine and l-tyrosine. It is the second direction that involves subsequent transformations to finally produce phenolic compounds in plant cells. This process starts with the chorismic acid transformation into prephenic acid (10). The latter undergoes either dehydration attended by decarboxylation or oxidative decarboxylation. In the first case, there is formed a phenylpyruvic acid (11) from prephenic acid and, in the other case, n-hydroxyphenylpyruvic acid (13). These keto acids are further aminated to give l-phenylalanine (12) and l-tyrosine (14), respectively.
The aforesaid transformations may, however, have another sequence. The amination may occur already at the stage of prephenic acid being first transformed into l-arogenic acid (15). Only afterwards does the molecule expose itself to dehydration followed by decarboxylation or to oxidative decarboxylation, resulting in l-phenylalanine and l-tyrosine. The construction of the benzene ring comes to an end with the formation of these two aromatic amino acids. The entire shikimate pathway is also completed, which, as a source of the said amino acids, represents actually one of the constituents of the primary cell metabolism. The specific secondary transformations leading to the biosynthesis of phenolic compounds begin only after this stage of metabolism; moreover, they spring from a single product of the shikimate pathway, l-phenylalanine [59, 60].
Shikimic acid in plants
Shikimic acid has been found to occur in many tissues of a variety of plants, with a sufficiently high percentage. Moreover, its content and accumulation in different tissues depends on the rate of metabolic processes taking place in them (Table 3) [61–66].
The quantitative distribution of shikimic acid in organs of various plants was studied by Hasegawa et al. [61]. The study results are listed in Table 4.
The variation of the shikimic acid concentration in a plant with advancing age was observed by many researchers. Henshaw et al. [62], while studying Iris pseudoacorus rhizome, found that the concentration of shikimic acid dropped from 739 μg/g of fresh mass in May to 545 μg/g in June, while the overall concentration of quinic acid remained primarily unchanged, although its increase was noted in March. Those authors reported that shikimic acid significantly decreases after leaf formation since it is involved in metabolic processes. In contrast, Neish [63] showed that the shikimic acid level in both annual and neoformed leaves of Picea pungens remained relatively constant during the study period of 4–6 months, which is probably associated with the high rate of the synthesis of aromatic compounds—the resin components—and, as a consequence, with the high rate of the shikimic acid synthesis [40].
Hulme [64] described an increase in the shikimic acid concentration in apple skin with advancing age. He also observed an increase in shikimic acid and a decrease in quinic acid in skins of pears being stored at 15 °C for 100 days. Hulme made an assumption that shikimic acid as an active metabolic intermediate occurs in free form in tissues wherein the metabolic processes decelerate. Whiting [65] observed that shikimic acid in gooseberry fruits was practically at a constant level from the unripe to the fully matured, which is in contrast to Hulme’s observations for apples and pears. Shikimic acid in the vine leaves vanishes at the ripening stage.
Thus, the shikimic acid concentration in plant organs is not constant and depends on the synthesis rate of aromatic amino acids which are precursors of polycyclic compounds. In addition, shikimic acid is observed to accumulate in those tissues wherein the metabolic processes are stopped or slow, such as storage tissue of seeds and fruits.
Shikimic acid was found to be present in cambia of Eucalyptus sieberiana and Eucalyptus regnans [66]. Hillis reported shikimic acid to be present in E. sieberiana leaves and indicated that the concentration reached its maximum when the leaves had grown to full size. The concentration drops with the further aging of the leaves, which is logically explained by a decrease in the synthesis rate of aromatics in tissues of the aging leaves.
The seasonal variation of the shikimic acid content in Ginkgo biloba and Pinus thunbergii was investigated by Hasegawa and Tateoka [67]. G. biloba had the maximum of shikimic acid in August, both in inner bark and in leaves. Thorough studies of G. biloba for shikimic acid percentage and its localization in different plant parts were conducted by Awang and Blumenthal [68].
Thus, the search for shikimic acid should be made in those tissues where the metabolic processes are stopped or where the metabolic process rate is high (storage tissue of plant seeds and fruits, roots, tubers, young vegetative parts during the intensive growth of a plant, and the like).
In a recent study, Enrich et al. [3] isolated shikimic acid (2.4–3.7% yields) from the seeds of Liquidambar styraciflua that grows galore in the eastern part of North America and in Mexico. Shikimic acid is also possible to isolate from Eucalyptus citriodora [69].
With the RP-HPLC technique, Bharathi et al. [70] managed to detect shikimic acid in a great many plants and analyze its localization in different plant organs. The results are listed in Table 5.
The highest percentage of shikimic acid was discovered in plants of the Illicium genus [70]; for example, the content of shikimic acid in I. religiosum fruits is over 24% on a dry basis [71]. The recovery of shikimic acid from Illicium verum fruits was maximum 7–10% [12, 72]. A great number of plants broadly distributed across Russia, particularly growing in Altai krai, were examined for shikimic acid by our research team. The results are listed in Table 6 showing the content of shikimic acid in separate parts of the plants studied [40].
As we have shown, the Siberian region is populated with plant raw materials only for the laboratory or at least for the preparative isolation of shikimic acid. Thus, the highest percentage of shikimic acid was detected in Pinus sylvestris needles from among the plant extracts examined. Nevertheless, the isolation of shikimic acid on a commercial scale from the needles seems inappropriate as well, although the low recovery of the product can pay for itself with interest due to cheap raw materials which, on top of that, go to waste, with multiple valuable products being lost.
From the literary sources, we are under the impression that all the plants suitable for isolating shikimic acid from their tissues (E. citriodora, L. styraciflua, plants of the Illicium genus, and a few others) grow in tropical countries and are difficult to cultivate in the temperature zone. Accordingly, productions of shikimic acid are confined to the regions where these plants grow (Southeast Asia, Japan’s south islands, south of the Northern America, and South America).
Thus, shikimic acid has been detected in a great plurality of plants, but only few kinds of plants are good for the isolation of shikimic acid. In order to meet the chemical industry demands for shikimic acid, it is therefore necessary to find an optimal approach to its isolation.
In connection with the worldwide rapid development of biotechnology, microbiology, genetics, and biochemistry, a breakthrough is being expected in the field of creating new microorganisms—hyperproducers of various substances, including shikimic acid. It seems to us that the future of producing organic acids lies precisely with microbiology.
Let us give a detailed consideration to the isolation techniques of shikimic acid from plant raw materials and microbes which exist today.
Shikimic acid isolation from plant and microbial sources
Shikimic acid isolation from plant raw materials
The first experiments on isolation and recovery of high-purity shikimic acid were conducted in the 1960s, twentieth century. For example, Weinstein et al. [73] have devised an effective technique for isolating C14-labelled shikimic acid from G. biloba L. for the purpose of studying the metabolism in plants, particularly for studying the shikimate pathway. The plant was kept for several days for metabolism in an atmosphere of radioactive carbon dioxide. The plant material was extracted with ethanol and water, and the extract was passed through a Dowex 50-X4 (H+ form) column to remove main impurities. The eluate was then passed through a column of Dowex 1-X8 (acetate form). The gradual elution with an aqueous acetic acid provided an excellent separation of shikimic and quinic acids. The final passage through the Dowex 1-X8 column gave compounds sufficiently pure for direct usage. Two hundred eighty-seven grams of fresh leaves gave 2.12 g of shikimic acid (0.74% on a wet basis) and 0.593 g of quinic acid.
The shikimic acid content in G. biloba L. is known to be no less than 4% based on wet leaves [73]. Accordingly, the aforesaid technique gave an extremely low yield (below 20%), which is due to the high shikimic acid loss during chromatographic purification.
Underhill et al. [74] employed a modified technique wherein Ginkgo and rose shoots were cultivated together in a radioactive atmosphere. The preparation of a crude extract from both plants provided a higher yield of shikimic acid (2.5% on a dry basis of Ginkgo leaves) than in case of using Ginkgo only.
These techniques are certainly of interest from the perspective of history, but are hardly applicable to the industrial-scale production of shikimic acid because they are multistage and lead to high product loss.
As described above, the basic source of shikimic acid is currently plants of the Illicium genus, in fruits of which the acid was discovered in 1885. The techniques for isolating shikimic acid from these plants’ fruits are being improved constantly. For instance, Adams et al. [71] extracted 900 g of Illicium anisatum seeds for 24 h in a Soxhlet extractor and performed a subsequent purification using a Solka-Floc anion exchange resin. Shikimic acid was obtained in 98% purity and >5% yield based on a dried fruit weight. Payne and Edmonds [36] modified and simplified the Adams’s method, though the shikimic acid yield was improved up to 7% on a dry basis.
About 25 g of I. anisatum seeds were ground to dust. The ground seeds were extracted in a Soxhlet extractor with 95% ethanol (125 ml) for approx. 2 h. The extract was evaporated to brown viscous oil (approx. 8.5 g) that was dissolved in water (145 ml) and heated to 80 °C. Five droplets of a 37–40% formaldehyde solution were added to the hot liquor which was fluxed for 5 min. After cooling down, the mixture was filtered to give an orange clear solution. The solution was passed through an anion exchange column (Amberlite IRA-400, acetate form, dry weight 25 g). Shikimic acid was eluted with acetic acid (185 ml of 25% aqueous acetic acid), and the yellow eluent collected. The eluent was concentrated; the solid residue was dissolved in methanol and heated for 10 min using activated carbon. The filtration and evaporation resulted in a dingy white solid residue (approx. 2.0 g). The solid residue was then recrystallized from methanol and toluene (or ethyl acetate), due to which the desired shikimic acid was obtained as a brilliant white, crystalline substance. The typical yield was between 0.60 and 1.74 g or from 30% to 85% based on an original content of shikimic acid in I. anisatum fruits (approx. 8.5%) [70].
The modified Adams’s and Payne’s methods are being employed in China for the commercial production of shikimic acid from plants of the Illicium genus. However, these commercial methods of producing shikimic acid are unfortunately good only for the tropical zone of the world because the Illicium genus plants are quite heat-loving and not suitable for cultivation in the temperature zone.
Li et al. [75] have developed an effective method for the isolation of shikimic acid from woody plants of the Liquidambar genus. Fruit coatings and leaves of the Liquidambar genus trees were air-dried for 24 h and then oven-dried at 65 °C. The milled material was further soaked in a tenfold volume of deionized water for 4 h. The deionized water extraction was repeated three times. The resultant extracts were passed through Amberlite IRA-400 (acetate form), and shikimic acid was eluted with 25% acetic acid. The eluate was concentrated and shikimic acid then crystallized from the mixed ethyl acetate and methanol. Thus, the method’s authors obtained shikimic acid in 98% purity and 70% yield based on an original content of shikimic acid in the feedstock (the original shikimic acid content in Liquidambar fruits and leaves is approx. 3–8% on a dry basis, depending on a species) [3].
The aforesaid method of producing shikimic acid is of sufficient interest for industry, but is unfortunately limited by the growth region of the heat-loving Liquidambar genus plants and is distinct in labor intensiveness related to the use of several sorbents. Nevertheless, the given method is being actively exploited in the Chinese industry for the production of shikimic acid from the Liquidambar genus plants.
Sui [76] outlines in his work a technique for isolating shikimic acid from P. sylvestris needles. Dried pine needles were ground and thrice extracted with a fivefold volume of water at 45 °C for 2 h with continuous stirring. To remove volatile organic acids, the aqueous extract was concentrated in a rotary evaporator in vacuum until the volume was twice decreased. Twenty grams of activated carbon was added to the concentrated extract which was then heated at 80 °C for 10 min with stirring. The solids were further withdrawn by filtration. The filtrate was deposited on an Amberlite IRA-900 anion exchange resin (acetate form), washed with water and methanol, and shikimic acid subsequently eluted with 2 M acetic acid in an aqueous solution. The eluate was further concentrated in the rotary evaporator until a yellow solid powder was produced. The resulting powder was dissolved in 95% methanol at 50–60 °C, followed by the addition of 2–5 g of activated carbon, and fluxed for 20 min. After filtration, the clear solution was concentrated to afford a viscous mass and cooled down for crystallization. The crystallization lasted 2–12 h. Coarse crystals were filtered, dissolved in 95% ethanol with heating, and then allowed to stand at 5 °C for recrystallization. The resultant white crystals were filtered and dried in a vacuum oven at 60 °C for 8 h, and the remaining liquid was again concentrated for crystallization, with the shikimic acid residual product being produced.
This technique is quite simple but not suitable for industry because the shikimic acid yield is low and constitutes only 70% of the original shikimic acid content in the needles. Moreover, the needles contain totally about 2% of shikimic acid on a dry basis.
Microbiological technique for shikimic acid
There exist a great number of publications on the isolation and purification techniques for shikimic acid using plant raw materials, but the creation of shikimic acid-hyperproducing microbial strains was begun only in the mid-1980s of the twentieth century when it became possible to study the genome of microorganisms, and the mechanism of shikimic acid formation and destruction in a cell (the shikimate pathway of metabolism) was completely investigated.
The first works on obtaining shikimic acid with the aid of microorganisms were conducted in the beginning of the 1950s of the last century [77], but pure shikimic acid was first possible to isolate from a culture broth only in 1962 [78]. The supernatant (after filtration of a culture broth resulted from Escherichia coli cultivation where labeled glucose served as a carbon source for the bacterium) was simply passed through a Dowex-1 column (acetate form) and eluted with ammonium acetate. The eluate was passed through a second column with the same sorbent, and shikimic acid was washed away with acetic acid. With this technique, Millican obtained shikimic acid in >95% purity, but in a low yield, about 30% of the original content of shikimic acid in the culture broth.
Millican’s method is quite interesting for research purpose, particularly to study metabolism in plants and microbes, but is ineffective for industrial application.
Iomantas et al. [79, 80] have created a number of Bacillus subtilis-based hyperproducing strains to produce shikimic acid. The yield of shikimic acid in B. subtilis was improved owing both to the proliferation of gene replicas responsible for the synthesis of a shikimate dehydrogenase enzyme and to the inhibition of genes responsible for the synthesis of a shikimate kinase enzyme. The strain created by Iomantas was cultivated in a bioreactor in a medium containing 150 g/l of glucose, 2 g/l of (NH4)2SO4, 3 g/l of NH4Cl, 3 g/l of KH2PO4, 0.4 g/l of MgSO4, 0.02 g/l of FeSO4, 15 g/l of yeast extract, and 10 mg/l of erythromycin at pH 7.0, 37 °C for 96 h, and at an air flow rate of 0.6 l/min. The culture broth was further centrifuged to separate cells and then concentrated. Shikimic acid from the concentrate was transferred to ethanol, and shikimic acid was further pre-purified by crystallization and recrystallization from ethanol. Shikimic acid was afterwards purified on ion exchange resins. This work is one of the first genetic engineering researches toward the development of new types of microorganisms. Using this technique, shikimic acid can be produced in a yield of about 14 g/l of culture broth, that is, 9.3% on a glucose basis (overall loss during purification was about 10%). But, on top of that, they could not have minimized the induction of dehydroshikimate and quinic acid into the culture broth. The said method can find its industrial application if used to resolve the problem of effective shikimic acid purification from dehydroshikimate and quinic acid.
Frost [81] have done a unique work on studying the mechanism of shikimic acid formation and destruction in the E. coli cell and have constructed a shikimic acid-hyperproducing E. coli strain defective in the shikimate dehydrogenase gene. This strain productivity was over 50 g of shikimic acid per liter of culture broth, constituting 10.4% on an original glucose basis. Besides shikimic acid, dehydroshikimate (approx. 12 g/l) and quinic acid (approx. 4 g/l) were actively produced, hindering further purification of shikimic acid. Previously, Frost and colleagues managed to create the E. coli SP1.1/pKD12.112 construct, a shikimic acid hyperproducer yielding 20.2 g of shikimic acid per liter of culture broth, 4.6 g/l dehydroshikimic acid, and 1.9 g/l quinic acid [82]. To separate shikimic acid, they used an IRA-400 ion exchange resin. The culture broth after cultivation of the E. coli SP1.1/pKD12.112 was centrifuged, concentrated on a rotary evaporator to a viscous mass, dissolved in methanol, and centrifuged again. The resultant alcoholic solution was stripped to dryness; the solid residue was dissolved in water, filtered, and deposited onto the IRA-400 resin (acetate form), then washed with water, and eluted with a 25% aqueous acetic acid. The eluate was concentrated and shikimic acid was crystallized from ethanol to give >98% purity and 85% yield (based on an original content of shikimic acid), but with a slight quantity of dehydroshikimate (below 0.5%). Based on the E. coli strains, an industrial technology has been developed to produce shikimic acid.
Johansson et al. [83] have constructed the E. coli strain W3110.shik1 modified in the aroL gene that encrypts the synthesis of the kinase II enzyme of shikimic acid. Both the constructed strain (W3110.shik1) and the control strain (W3110) were studied by cultivation in a chemostat to determine the products’ yields under well-defined growth conditions. Both glucose-limited and phosphate-limited conditions were under study. The latter conditions were mainly selected as a means of investigating glucose-rich conditions which, as shown by Johansson, resulted in a higher shikimic acid yield. The extracellular metabolites attained directly through the shikimate pathway were produced by W3110.shik1 only. With limited phosphate, the shikimic acid yield was two times higher than that for limited carbon. The dehydroshikimic acid yield was about one third of the shikimic acid yield, whereas there were no detectable amounts of both quinic acid and gallic acid. The loss of shikimic acid via dehydroshikimate under carbon-limited conditions may in fact be higher than the measured concentration of dehydroshikimate because dehydroshikimate may be destructible in the presence of phosphate. Thus, increasing the phosphate content in the culture broth during cultivation of W3110.shik1 led to a higher yield of shikimic acid and low yields of by-products from the shikimate pathway. However, an increased cell killing with the concurrent formation of acetate was also observed, which may lead to problems during the purification of shikimic acid when commercially produced from the E. coli strain W3110.shik1.
The recovery of shikimic acid from the E. coli strain W3110.shik1 was about 12 g/mol of glucose, which is a sufficiently low value for hyperproducing strains. The work of Johansson et al. is quite interesting from scientific and practical points of view, and the findings are of great importance for the industrial cultivation of shikimic acid-producing E. coli strains.
Van der Does et al. [84] reported an intriguing technique for the purification of shikimic acid from a culture broth after cultivation of shikimic acid-producing microorganisms. A culture broth containing shikimic acid was filtered through a 20-nm membrane filter to separate cells, then concentrated and fluxed at reduced pressure and 108 °C for 4 h. The resultant aqueous mixture was acidified with sulfuric acid to pH 2.6 and filtered; the filtrate was lyophilized. Shikimic acid from the lyophilizate was acetone-extracted at room temperature. Activated carbon was added to the extract and the mixture stirred for 15 min. The mixture was filtered and the wet residue washed with acetone. The filtrates were combined. The solution was evaporated at 30–35 °C, cooled to room temperature, a small amount of shikimic acid seed was added, and the mixture was stored for 2–3 days at 0 °C. Shikimic acid crystals precipitated from the extract and were further dissolved in ethanol and recrystallized. After recrystallization, shikimic acid had about 96% purity and over 80% yield based on the original shikimic acid per liter of culture broth.
The aforesaid procedure for the purification of shikimic acid is of interest from the perspective of industrial production of shikimic acid from culture broths because it requires no expensive sorbents and is easy to run.
Enzymatic technique for shikimic acid
A curious method for the enzymatic synthesis of shikimic acid from quinic acid was described by Adachi et al. [85–89]. Dried cells or the membrane fraction of Gluconobacter oxydans IFO 3244 containing quinate dehydrogenase and 3-dehydroquinate dehydrotase were incubated with quinic acid at pH 3.0–10.0 and 30 °C with continuous stirring for 20 h. The reaction gave 3-dehydroshikimate in about 80% yield. The reaction mixture was deposited onto a Dowex resin and eluted with aqueous acetic acid. Fractions containing 3-dehydroshikimate were neutralized with alkali and lyophilized. 3-Dehydroshikimate was then dissolved in a phosphate buffer at pH 7.0, followed by the addition of NADP, NADP-dependent d-glucose dehydrogenase and NADP-dependent shikimate dehydrogenase (the enzymes were obtained from G. oxydans IFO 3244), and excess d-glucose.
This technique is of interest from the perspective of the laboratory recovery of shikimic acid, but is labor-intensive for industrial application and requires a permanent inexpensive source of quinic acid.
Conclusions
Thus, there are four general approaches to producing shikimic acid: (1) synthetic approach oriented on the chemical synthesis of shikimic acid (omitted in the present review); (2) an approach where plants serve as shikimic acid sources; (3) an approach where microorganisms are shikimic acid sources; (4) enzymatic approach where shikimic acid synthesis involves enzymes.
The enzymatic technique of producing shikimic acid from quinic acid can be attractive only as a laboratory-scale synthesis. This method requires a cheap source of quinic acid; it is multistage and needs the cultivation of microorganisms and isolation of enzymes therefrom. It is therefore very labor-intensive, which will cause a rise in price for the product when produced on a commercial scale. Hence, the enzymatic method cannot be applicable to the industrial production of shikimic acid at the present stage of development of biotechnology in the world.
All the techniques to produce shikimic acid by its isolation from plants have a number of limitations for use in industry. Their main drawback is that they are confined to the region where the plants grow. Moreover, the isolation of shikimic acid from plants is limited by a certain season of year when the shikimic acid level is sufficient for industrial process. In contrast to the methods that utilize plant parts as raw materials, the culture broth-based techniques for isolating shikimic acid are free from such limitations. Disadvantages of the microbiological methods include high labor intensiveness bound up with cultivation of microorganisms and complexities in scaling up. Nevertheless, they have an undisputable advantage over the methods utilizing plants as raw materials, that is, high yields of shikimic acid and a relative purity and homogeneity of raw material. Microbes do not require huge areas for seeding, and their rates of growth and metabolism have no parallel in the vegetable world. The isolation of shikimic acid from culture broths is simple and requires no expensive reagents and sorbents.
In summary, the following conclusions can be drawn with respect to the applicability of the isolation techniques to commercial-scale production of shikimic acid:
-
The enzymatic synthesis of shikimic acid requires a cheap source of quinic acid, expensive reagents, separation of highly purified enzymes, and is multistage. The product yield is low. It is not applicable to the industrial-scale production of shikimic acid.
-
The isolation of shikimic acid from plants is confined to a certain season of year and to a plant growth place. The product yield is subject to the quality of raw materials. It is multistage and employs costly sorbents. This approach is applicable to the industrial production of shikimic acid in growth locations of a plant having a high percentage of the product.
-
The isolation of shikimic acid from culture broths demands the observance of cleanness while cultivating microorganisms. This technique is characterized by the less number of stages in the production of shikimic acid as compared with those using plant raw materials. Requiring no expensive reagents and sorbents, they result in high yields of the product and are suitable for the commercial-scale production of shikimic acid.
The optimal techniques to produce shikimic acid on a commercial scale are therefore those which utilize different strains of hyperproducing microorganisms. The microbiological methods will allow shikimic acid to be produced at minimal costs and in high yields.
References
Brown SA (1964) Biochemistry of phenolic compounds. Academic, New York
Bradley D (2005) Nat Rev Drug Discov 4:945
Enrich LB, Scheuermann ML, Mohadjer A, Matthias KR, Eller CF, Newman MS, Fujinaka M, Poon T (2008) Tetrahedron Lett 49:2503
Lingens F (2003) Angew Chem Int Edit 7:350
Mair H (2000) Patent US006130354
Zhang Y, Liu A, Ye ZG, Lin J, Xu LZ, Yang SL (2006) Chem Pharm Bull 54:1459
Luhong T, Hong X, Yang S, Liying Q, Dongqun C, Chao D, Wei C (2009) Appl Biochem Biotechnol 158:408
Huang F, Xiu Q, Sun J, Hong E (2002) J Cardiovasc Pharmacol 39:262
Jiang S, Singh G, Boam DJ, Coggins JR (1999) Tetr Assym 10:4087
Song C, Jiang S, Singh G (2001) Tetr Assym 42:9069
Gibson JM, Thomas PS, Thomas JD, Barker JL, Chandran SS, Harrup MK, Draths KM, Frost JW (2001) Angew Chem Int Ed 40:1945
McCrindle R, Overton KH, Raphael RA (1960) J Chem Soc, p 1560
Wada H, Kido T, Tanaka N, Murakami T, Saiki Y, Chen CM (1992) Chem Pharm Bull 40:2099
Snyder D, Rapoport H (1973) J Am Chem Soc 95:7821
Shinada T, Yoshida Y, Ohfune Y (1998) Tetrahedron Lett 39:6027
Achenbach H, Schwinn A (1995) Phytochemistry 38:1037
Salamon II, Davis BD (1953) J Am Chem Soc 75:5567
Weiss U, Ziffer H (1963) J Org Chem 28:1248
Bressi JC, Verlinde CLMJ, Aronov AM, Shaw ML, Shin SS, Nguyen LN, Suresh S, Buckner FS, Van Voorhis WC, Kuntz ID, Hol WGJ, Gelb MH (2001) J Med Chem 44:2080
Smissman EE, Suh JT, Oxman M, Daniels R (1962) J Am Chem Soc 84:1040
Evans DA, Barnes DM (1997) Tetrahedron Lett 38:57
Hill RK, Newkome GR (1969) J Am Chem Soc 91:5893
Coblens KE, Muralidharan VB, Ganem B (1982) J Org Chem 47:5041
Luthe C, Zamir LO (1983) Tetrahedron Lett 24:4409
Mirza S, Harvey J (1991) Tetrahedron Lett 32:4111
Brazdova B, Tan NS, Samoshina NM, Samoshin VV (2009) Carbohyd Res 344:311
Kranich R, Busemann AS, Bock D, Schroeter-Maas S, Beyer D, Heinemann B, Meyer M, Schierhorn K, Zahlten R, Wolff G, Aydt EM (2007) J Med Chem 50:1101
Reyes-Chilpa R, Estrada-Muñiz E, Apan TR, Amekraz B, Aumelas A, Jankowski CK, Vázquez-Torres M (2004) Life Sci 75:1635
Shende J, McCullough KJ, Boualem M, Gurdial S, Richard HW (1997) J Chem Soc Perkin Trans 1:1805
Perkins HJ, Aronof TS (1959) Can J Biochem Physiol 37:149
Zaprometov MN (1961) Biokhimiya 26:597
Yoshida S, Hasegawa M (1957) Arch Biochem Biophys 70:377
Gaitonde MK, Gordon MW (1958) J Biol Chem 230:1043
Saslaw LD, Waravdekar VS (1960) Biochim Biophys Acta 37:367
Millican RC (1963) Anal Biochem 6:181
Payne R, Edmonds M (2005) J Chem Educ 82:599
Schuster MC, Mann DA, Buchholz TJ, Johnson KM, Thomas WD, Kiessling LL (2003) Org Lett 5:140
Nonaka G, Ageta M, Nishioka I (1985) Chem Pharm Bull 33:96
Rukovodstvo po metodam kontrolya i bezopasnosti biologicheski aktivnykh dobavok k pishche (in Russian). Rukovodstvo. R 4.1.1672-03 (Utverzhdeno Glavnym gosudarstvennym vrachem RF 30.06.2003)
Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA (2011) Issledovanie soderzhaniya shikimovoi kisloty v nekotorykh rasteniyakh Altaiskogo kraya (Study of the shikimic acid content in some plants of Altai krai). Khimiya Rastitel'nogo Syr'ya (In Russian) 1:119–122
Bresnahan G, Manthey F, Howatt K, Chakraborty M (2003) J Agric Food Chem 51:4004
Silva B, Andrade P, Mendes G, Seabra R, Ferreira M (2002) J Agric Food Chem 50:2313
Dainiak MB, Galaev IY, Mattiasson B (2002) J Chromatogr A 942:123
García Romero E, Sánchez Muñoz G, Martín Alvarez PJ, Cabezudo Ibáñez MD (1993) J Chromatogr A 655:111
Tusseau D, Benoit C (1987) J Chromatogr A 395:323
Mardones C, Hitschfeld A, Contreras A, Lepe K, Gutiérrez L, Baer D (2005) J Chromatogr A 1085:285
Nagels L, Debeuf C, Esmans E (1980) J Chromatogr A 190:411
Heimler D, Pieroni A (1994) Chromatographia 38:475
Molnár-Perl I, Vasanits A, Horváth K (1998) Chromatographia 48:111
Horváth K, Molnár-Perl I (1998) Chromatographia 48:120
Jaroszynska J (2003) Anal Bioanal Chem 377:702
Liu HC, Li QW, Tang LB (2007) J Zhejiang Univ Sci B 8:272
Ibarz MJ, Ferreira V, Hernandez-Orte P, Loscos N, Cacho J (2006) J Chromatogr A 1116:217
Eykman JF (1885) Rec Trav Chim 4:32
Wallace KK, Reynolds KA, Koch K, McArthur HAI, Brown MS, Wax RG, Moore BS (1994) J Am Chem Soc 116:11600
Flores N, Xiao J, Berry A, Bolivar F, Valle F (1996) Nature Biotechnol 14:620
Herlt AJ, Rickards RW, Wu JP (1985) J Antibiot 38:516
Jones AL, Keller U (1997) In: Strohl WR (ed) Biochemistry and genetics of actinomycin production. Marcel Dekker, New York
Wilson DJ, Patton S, Florova G, Hale V, Reynold KA (1998) J Ind Microbiol Biot 20:299
Bentley R (1990) Biochem Mol Biol 25:307
Hasegawa M, Nakagawa T, Yoshida S (1957) J Jap For Soc 39:159
Henshaw GG, Coult DA, Boulter D (1962) Nature 194:579
Neish AC (1958) Can J Botany 36:649
Hulme AC (1956) Nature Lond 178:991
Whiting GC (1957) Nature 179:531
Hillis WE, Carle A (1958) Holzforschung 12:136
Hasegawa M, Tateoka T (1960) J Jap For Soc 42:224
Awang DVC, Blumenthal M (2006) HerbalGram 70:58
Anet EF, Birch AJ, Massy-Westropp RA (1957) Aust J Chem 10:93
Bharathi A, Wang YH, Smillie TG, Khan IA (2009) Chromatographia 69:307
Adams H, Bailey N, Brettle R, Cross R, Frederickson M, Haslam E, MacBeath F, Davies G (1996) Tetrahedron 52:8565
Wang XQ, Guo YD, Yang CB (2001) Zhongguo Zhongyao Zazh (Chinese) 26:447
Weinstein LH, Porter CA, Laurencot HJ (1962) Contrib Boyce Thompson Inst 21:439
Underhill EW, Watkin JE, Neish AC (1957) Can J Biochem Physiol 35:219
Li S, Yuan W, Wang P, Zhang Z, Zhang W, Ownby S (2007) Processes for the extraction and purification of shikimic acid and the products of such processes. Patent US2007/0161818
Sui R (2008) Chem Eng Technol 31:469
Mitsuhashi S, Davis BD (1954) Biochim Biophys Acta 15:268
Millican RC (1962) Biochim Biophys Acta 57:407
Iomantas YAV, Abalkina EG, Polanuer BM, Yampolskaya TA, Bachina TA, Kozlov YI (1999) Sposob polucheniya shikimovoi kisloty. Patent RU2206612C2 (in Russian)
Iomantas YAV, Abalkina EG, Polanuer BM, Yampolskaya TA, Bachina TA, Kozlov YI (2002) Method for producing shikimic acid. Patent US6,436,664
Frost JW (2007) Methods and materials for the production of shikimic acid. Patent US2007/087424
Draths KM, Knop DR, Frost JW (1999) J Am Chem Soc 121:1603
Johansson L, Lindskog A, Silfversparre G, Cimander C, Nielsen K, Lide G (2005) Biotechnol Bioeng 92:541
Van der Does T, Booij J, Kers EE, Leenderts EJAM, Sibeijn M, Agayn V (2002) Process for the recovery of shikimic acid. Patent WO02/06203
Adachi O, Ano Y, Toyama H, Matsushita K (2006) Biosci Biotechnol Biochem 70:2579
Adachi O, Tanaspawat S, Yoshihara N, Toyama H, Matsushita K (2003) Biosci Biotechnol Biochem 67:2124
Adachi O, Tanaspawat S, Yoshihara N, Toyama H, Matsushita K (2003) Biosci Biotechnol Biochem 67:2112
Adachi O, Moonmangmee D, Toyama H, Yamada M, Shinagawa E, Matsushita K (2003) Appl Microbiol Biotechnol 60:643
Adachi O, Moonmangmee D, Shinagawa E, Toyama H, Yamada M, Matsushita K (2003) Biosci Biotechnol Biochem 67:10
Metzenberg RL, Mitchell HK (1958) Biochem J 68:168
Hathway DE (1956) Biochem J 63:380
Hennig K, Burkhardt R (1958) Weinberg Keller 5:542
Gordon HT, Thornburg W, Werun LN (1956) Anal Chem 28:849
Simonart P, Wiaux A (1960) Nature 186:78
Hillis WE, Carle A (1960) Biochem J 74:607
Davis BD, Mingioli ES (1953) J Bacteriol 66:129
Coulson CB, Evans WC (1958) J Chromatog 1:374
Carr JG, Pollard A, Whiting GC, Williams AH (1957) Biochem J 66:283
Holmes GW, Kurth EF (1961) Tappi 44:893
Tachi I, Sato A (1960) Bull Agr Chem Soc Japan 24:633
Hattori S, Yoshida S, Hasegawa M (1958) Arch Biochem Biophys 74:480
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bochkov, D.V., Sysolyatin, S.V., Kalashnikov, A.I. et al. Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources. J Chem Biol 5, 5–17 (2012). https://doi.org/10.1007/s12154-011-0064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12154-011-0064-8