Abstract
Objective
Dopamine transporter (DAT) imaging such as 123I-ioflupane (123I-FP-CIT) SPECT is a useful tool for the diagnosis of parkinsonism and dementia. The Southampton method is the quantitative method for evaluating 123I-FP-CIT SPECT and is less affected by the partial volume effect of the striatum. The method may be vulnerable to contamination by low-uptake areas of cerebrospinal fluid in whole brain, and the threshold of voxel value (threshold method, TM) was developed to correct the contamination. The purpose of this study is to evaluate the TM in the patients with neurological disease.
Methods
We studied 99 subjects, including 39 patients with Alzheimer’s disease (AD), 15 patients with Parkinson’s disease (PD) and 10 patients with dementia with Lewy bodies (DLB). Each subject had undergone 123I-FP-CIT SPECT. We calculated the SBR with and without the TM. The SBR laterality was assessed using the asymmetry index (AI). We investigated the relationship between the SBR change with TM and brain atrophy, which were assessed using Evans index (EI), sylvian index (SI) and cerebral atrophy index (CAI). Cutoff value for EI was 0.3, and cutoff values for SI and CAI were the first quartile, respectively.
Results
The SBR with TM was 0.53 percentage points lower than the SBR without TM overall (p < 0.01). Positive and negative reversal of AI increased with age. The rate of the SBR change with TM was tended to be lower in groups with brain atrophy. The number of voxels excluded by TM in striatal volumes of interest (VOIs) was larger with high groups for EI, SI and CAI than in low groups. The number of voxels excluded using TM in reference VOIs was related to SI.
Conclusions
The SBR was decreased using TM. The effect of TM on the SBR tended to be small in the subjects with severe brain atrophy. The effect of brain atrophy in the TM is larger in the striatal VOIs than in the reference VOIs. Even if quantitative analyses are available, visual assessment of 123I-FP-CIT SPECT is essential for diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Dopamine transporter (DAT) imaging modalities such as 123I-ioflupane (123I-FP-CIT) single-photon emission computed tomography (SPECT) were approved in 2004 in Europe and in 2014 in Japan. Such imaging offers a useful tool for distinguishing degenerative parkinsonian syndrome (PS) from other forms of PS and Alzheimer’s disease (AD) [1, 2] and has been incorporated into diagnostic criteria in recent guidelines.
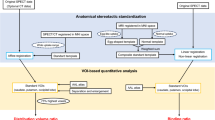
Using positron emission tomography (PET), we can recognize the contours of the striatum and cerebellum in the DAT images. We used to place regions of interest (ROIs) in the striatum and the cerebellum and semi-quantitated the density of DAT with the uptake ratio index [2]. Due to the low resolution of SPECT, however, it is difficult to recognize these contours in the 123I-FP-CIT images, such as putamen with low accumulation in the patients with PS. Therefore, a method for detecting the outline of the striatum using spatial normalization was developed, such as DaTQUANT (GE Healthcare, Little Chalfont, UK). This method is concerned with the accuracy of spatial normalization in the putamen where the accumulation declines in PS. The occipital lobe is used for the reference area in DaTQUANT, although some papers were reported that the cerebral metabolism and blood flow of the occipital lobe decreased in Lewy body disease. On the other hand, Tossici-Bolt et al. [3] proposed the Southampton method for evaluating 123I-FP-CIT SPECT. In the Southampton method, we use large volume of interest (VOI) including all the striatum. We can place the VOI for striatum, even if we cannot recognize accurate contours of striatum. Although the striatum has a pointed and narrow shape, we can quantitate the density of DAT as the specific binding ratio (SBR) without consideration of striatal partial volume effect.
In clinical situations, however, some DAT images do not match the asymmetry of the SBR calculated by the Southampton method and visual assessment by experts. We think that most of the troublesome images with discrepancies involve enlarged sulci and ventricles due to severe brain atrophy. Discrepancies may be due to the contamination by low-uptake areas outside the brain such as sulci and ventricles in pentagonal VOIs for striatum and ellipsoid VOIs for reference (Fig. 1). Mizumura et al. [4] recently reported that the SBR with the Southampton method is vulnerable to contamination by low-uptake areas of cerebrospinal fluid in whole brain. They also emphasized the contamination could be corrected by the threshold of voxel value (threshold method, TM) as well as mask method using magnetic resonance imaging (MRI) segmentation. Using TM in the Southampton method, we can detect voxels for the low-uptake areas and may calculate the accuracy of SBR. The difference in the effect of brain atrophy in between the pentagonal VOI for striatum and the ellipsoid VOI for reference is not unknown. This could be tested by comparing the SBR with the severity of atrophy, such as enlargement of the lateral ventricles and sylvian fissure. The purpose of this study was to verify fluctuations in the SBR with or without the TM.
Illustrations of normal brain (a) and atrophic brain (b). Compared with normal brain (a), the atrophic brain (b) shows a larger sulcus and ventricles. In the atrophic brain, both pentagonal volumes of interest (VOIs) for striatum and ellipsoid VOIs for reference contain many voxels from sulcus and ventricles that had no uptake
Methods
Data from 99 consecutive subjects who had undergone DAT SPECT between August 2015 and July 2016 were retrospectively analyzed. MRI images were available in 93 subjects, and CT images were available in other six subjects. Clinical characteristics are summarized in Table 1. Clinical information obtained included age, sex and diagnosis. This study was approved by the institutional review board at Nippon Medical School Musashi Kosugi Hospital.
DAT imaging was performed using a dual-headed E.CAM gamma camera (Toshiba, Tokyo, Japan) with low–medium-energy general purpose (LMEGP) collimators. Each subject received an intravenous injection of 167 MBq of 123I-FP-CIT. Starting 4 h post-injection, a static scan was performed for 28 min. The radius of SPECT rotation was fixed at 15 cm and made a circular orbit. Projection images were obtained in 360° by each 120° head at 4° per step. The matrix size was 128 × 128, and the magnification factor of 1.45 resulted in a pixel size of 3.30 mm. Counts were acquired within a 30% symmetrical energy window centered at 159 keV. Data were reconstructed using filtered back-projection with a Butterworth filter (order 8; cutoff frequency 0.45 cycles/cm). The Chang method was used for attenuation correction without scatter correction: µ = 0.070/cm [4].
We calculated the SBR with and without TM using a medical image processing application DaTView version 8.0.0 in an application package (AZE VirtualPlace Hayabusa, AZE Co., Tokyo, Japan) [4]. The pentagonal VOI was first placed automatically by extracting highly integrated voxel in the DaTView, after which a neurologist (T. H.) visually corrected the position. For the placing of the reference VOI, we used the automatic outer contour extraction method (AOCEM) of DaTView. First, each SPECT mage was processed with a Gaussian filter of full width at half maximum (FWHM) 16 mm, which is the default parameter of DaTView. Secondly, a histogram of radioactivity was produced weighting the factors of the distance from the image center and an inflection point was detected between the radioactivity outside and inside of brain. Thirdly, the SPECT image was binarized with the inflection point as a discrimination threshold and a contour of the brain was determined. The threshold we used in this study was mean-1SD in the reference VOI, which is the value recommended by Mizumura et al. [4].
Magnetic resonance imaging (MRI) was acquired using a 1.5-T scanner (Intera, Philips, Eindhoven, The Netherlands). Computed tomography (CT) was performed with a 5-mm slice thickness, without constant at 120 kV and 250 or 350 mA (Aquilion Prime 80-row scanner, Toshiba, Tokyo, Japan, and Revolution HD 64-row scanner, GE Healthcare, Wisconsin, USA). We assessed cerebral atrophy in the AD group with normal DAT image and PS group with decreased DAT including Parkinson’s disease (PD), dementia with Lewy bodies (DLB) and progressive supranuclear palsy (PSP). One patient with PSP was excluded in the analysis of comparison with degree of atrophy in the morphological images, because his MRI was performed at other hospital and we could not measure the extent of his brain atrophy. We measured five factors as shown in Fig. 2 using the MRI and CT in the two groups and calculated three indexes: Evans index (EI), sylvian index (SI) and cerebral atrophy index (CAI). EI is an established index used for the diagnosis of normal pressure hydrocephalus [5] and is defined as follows:
where a is the transverse diameter of the anterior horns and b is the maximum transverse diameters of the brain (Fig. 2a). The cutoff value for EI was 0.3, because Evans reported the value above 0.3 indicates definite ventricular enlargement. We defined SI as the ratio of the sum of bilateral maximum heights of the sylvian fissure to the maximum internal diameter of the brain using the nearest MRI or CT image (Fig. 2b) [6]. SI was calculated as follows:
where c and d are the maximum heights of the sylvian fissure and e is the maximum transverse diameters of the brain. The cutoff value for SI was defined the first quarter as the abnormal value in the patients with AD and PS. We also defined CAI as sum of EI and SI:
The cutoff value for CAI was also defined the first quarter as the abnormal value in the patients with AD and PS.
The SBR laterality often shows the asymmetrical symptoms in patients with parkinsonism. To examine whether TM effects the SBR laterality, we defined asymmetry index (AI) as follows:
where SBRrt is the right SBR and SBRlt is the left SBR. This was calculated in patients with PS whose SBR was low and in patients with AD whose brain was atrophic. To assess the effect of TM on the SBR, we defined the SBR TM ratio as follows:
where SBRNC is the mean of the bilateral SBR without TM and SBRTM is the mean of the bilateral SBR with TM. We also defined the striatal, reference and total TM ratio to investigate the effects of TM on the volume of striatal, reference and total VOIs, as follows:
where VNC st represents the number of voxels of bilateral striatal VOIs without TM, VTM st is the number of voxels of bilateral striatal VOIs with TM, VNC ref is the number of voxels of reference VOIs without TM, VTM ref is the number of voxels of reference VOIs with TM, VNC to is the number of total voxels without TM and VTM to is the number of total voxels with TM, measured by referring to DaTView results. We also calculated the total number of voxels as the sum of striatal and reference VOIs.
Statistical analysis
Statistical computations were performed using the JMP Pro version 14.2.0 software package (SAS Institute, Cary, NC). The SBRs with and without TM were initially compared for each disease and overall. Next, we compared AI with and without TM for each disease using paired t-tests. We examined the relationship between the SBR TM ratio and brain atrophy in AD and PS. We counted voxels excluded using TM and used the same test to examine the relationship with brain atrophy. If the variance had a significant difference between groups with Bartlett’s test, Welch’s t test was used to the above examinations. If not, unpaired t-tests were used instead. Results were considered significant at the level of p < 0.05.
Results
The mean SBR was 3.91 with TM and 4.44 without TM (p < 0.0001, paired t-test, Table 2). Similar results were shown for most diseases except for PSP and FTLD (Table 2).
Five subjects showed reversal of the larger SBR side in AD, one person in DLB, one person in PD, one person among healthy individuals and one person among others. In the PD and DLB groups, the asymmetry of the SBR did not change with TM or not. Positive and negative reversal of the AI was increased with age (75.1 ± 11.0 vs 81.8 ± 5.4, p < 0.01, Welch’s t-test).
EI, SI and CAI were calculated in 39 patients with AD and 27 patients with PS. The first-quartile cutoff for SI and CAI was 0.15 and 0.47, respectively. Table 3 shows the number of cases in each group. The SBR TM ratio tended to be lower in the high-EI group than in the low-EI group, though not significant (p = 0.65, Welch’s t-test). There was also no relationship between the SBR TM ratio and SI (p = 0.31, unpaired t-test) and CAI (p = 0.39, Welch’s t-test). In patients with AD, the SBR TM ratio tended to be lower in the high-EI group than in the low-EI group, though not significant (p = 0.10, unpaired t-test). The SBR TM ratio of AD patients was significantly lower in the high-SI group than in the low-SI group (p < 0.05, unpaired t-test). The ratio was also significantly lower in the high-CAI group than in the low-CAI group (p < 0.05, unpaired t-test). In patients with PS, no relationship was evident between the SBR TM ratio and EI (p = 0.95, Welch’s t-test), SI (p = 0.63, unpaired t-test) and CAI (p = 0.89, unpaired t-test).
Striatal TM ratio in the high groups for EI, SI and CAI was higher than in the low groups (p < 0.05, unpaired t-test, Table 4). Reference TM ratio was significantly lower in the high-SI group than in the low-SI group (p < 0.01, unpaired t-test), although the ratio had no relationship with EI (p = 0.147, unpaired t-test) and CAI (p = 0.125, unpaired t-test). Total TM ratio in the high groups for EI, SI and CAI was higher than in the low groups (p < 0.05, unpaired t-test). In the AD patients, the striatal and total TM ratio in the high groups for EI, SI and CAI was higher than in the low groups. The reference TM ratio was significantly lower in the high-SI group than in the low-SI group, although the ratio had no relationship with EI and CAI. In the PS patients, the striatal, reference and total TM ratio had no relationship with EI, SI and CAI.
The total number of voxels was significantly lower in the high-SI group than in the low-SI group (p < 0.005, unpaired t-test), but showed no relationship with EI (p = 0.15, unpaired t-test) and CAI (p = 0.17, unpaired t-test). In the AD patients, the total number of voxels was also lower in the high-SI group than in the low-SI group, but had no relationship with EI and CAI. In the PS patients, the total number of voxels had no relationship with EI, SI and CAI.
Discussion
Our data showed that the SBR of 123I-FP-CIT decreased by 0.53 when TM excluded contamination in low-uptake voxels of cerebrospinal fluid of brain (Table 2). The SBR calculated with TM was lower than without TM in almost all diseases. Five subjects showed a change in the laterality of the SBR with or without TM and were significantly older than the others. This result is convincing because aging is strongly associated with brain atrophy. It is a serious problem that the asymmetry of the SBR is reversed due to the difference in analysis methods. This result indicates that visual assessment of 123I-FP-CIT SPECT is essential for diagnosis even if quantitative analyses are available.
Table 3 shows, however, that the changes in the SBR with or without TM tended to be small in the groups with severe brain atrophy measured as EI, SI and CAI. Especially in AD patients, the changes were significantly larger in the groups with lower SI and CAI than with higher. We then examined the change in the number of voxels with or without TM in striatal and reference VOIs (Table 4). As a matter of course, the total TM ratio was larger in the high group for EI, SI and CAI. That is, in severe atrophy, many voxels were removed by TM. The striatal TM ratio was also the same as the total TM ratio. However, the reference TM ratio for SI showed the opposite result of the striatal and total TM ratio. The reason is that, as the data of the total number of voxels show, the reference VOI was smaller when the sylvian fissure was enlarged. In this study, we used the automatic AOCEM for the placing of the reference VOI. In this method, the spatially normalized DAT images were produced with a Gaussian filter to create histograms for calculating the discrimination threshold. In the DAT images with enlarged cerebral sulci, the contour of the brain may shift to the center using AOCEM. The reference VOI for the DAT image with enlarged cerebral sulci moved inward from the original contour of the brain, therefore, and the voxels that should have been removed by TM must have decreased in the reference VOI. Then, the total number of voxels was smaller in the high-SI group than in the low-SI group, and the changes in the SBR by TM were small in the subjects with brain atrophy.
Table 4 also shows that the effect of brain atrophy in the TM is larger in the striatal VOIs than in the reference VOIs. The striatal VOIs always contain lateral ventricles and may contain parts of enlarged sylvian fissure of atrophic brain (Fig. 1). On the other hand, the reference VOIs always contain lateral ventricles and cerebral sulci such as sylvian fissure. As mentioned above, the volume of the reference VOI decreases with severe brain atrophy and removal effect by TM is reduced, when we use outer contour extraction method. The SBR of the Southampton method [3] was calculated as follows:
where CtVOI is the total counts in the striatal VOI, cr is the mean counts per pixel in the reference VOI, VVOI is the volume of the striatal VOI and Vs is the volume of the striatum (= 11.2 mL). Therefore, cr, which is the denominator, has a greater effect on the SBR than the numerator even if the change in the reference VOI is small. We should consider the impact of brain atrophy in VOI measurement. TM is suitable for solving this issue. Buchert et al. [7] first reported the influence of dilated CSF space on the SBR. The Southampton method uses a whole brain without striata as a reference region that has a limit because the region includes dilated ventricle and interhemispheric voxels. Unlike the conventional Southampton method, TM can calculate accurate the SBR which may decrease discrepancies between visual assessment and the SBR. Decrease in the SBR by about 0.53 percentage points leads to abnormal level of the SBR and may contribute the diagnosis of the degenerative parkinsonian syndrome as well.
Several limitations to the present study must be considered. This was a single-center, retrospective study, and the sample size was thus small. We did not investigate the duration or severity of disease. Further studies are needed to clarify the involvement of atrophy in the SBR.
Conclusions
In this study, we investigated the effects of brain atrophy in the SBR of the Southampton method. Using TM to exclude contamination in low-uptake voxels of cerebrospinal fluid of brain, the SBR was decreased. The effect of TM on the SBR tended to be small in the subjects with severe brain atrophy. The effect of brain atrophy in the TM is larger in the striatal VOIs than in the reference VOIs. Even if quantitative analyses are available, visual assessment of 123I-FP-CIT SPECT is essential for diagnosis.
Abbreviations
- 123I-FP-CIT:
-
N-v-fluoro-propyl-2b-carbomethoxy-3b-(4-123I-iodophenyl)nortropane
- SPECT:
-
Single-photon emission computed tomography
- DAT:
-
Dopamine transporter
- SBR:
-
Specific binding ratio
- TM:
-
Threshold method
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- DLB:
-
Dementia with Lewy bodies
- PS:
-
Parkinsonian syndrome
- AI:
-
Asymmetry index
- EI:
-
Evans index
- SI:
-
Sylvian index
- VOI:
-
Volume of interest
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- MBq:
-
Megabecquerel
- PSP:
-
Progressive supranuclear palsy
- FTLD:
-
Frontotemporal lobar degeneration
References
Plotkin M, Amthauer H, Klaffke S, Kühn A, Lüdemann L, Arnold G, et al. Combined 123I-FP-CIT and 123I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transm. 2005;112(5):677–92.
Mishina M, Ishii K, Suzuki M, Kitamura S, Ishibashi K, Sakata M, et al. Striatal distribution of dopamine transporters and dopamine D2 receptors at different stages of Parkinson’s disease–A CFT and RAC PET study. Neuroradiol J. 2011;24(2):235–41.
Tossici-Bolt L, Hoffmann SMA, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33(12):1491–9.
Mizumura S, Nishikawa K, Murata A, Yoshimura K, Ishii N, Kokubo T, et al. Improvement in the measurement error of the specific binding ratio in dopamine transporter SPECT imaging due to exclusion of the cerebrospinal fluid fraction using the threshold of voxel RI count. Ann Nucl Med. 2018;32(4):288–96.
Evans WA. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatr. 1942;47(6):931–7.
Tan U, Kutlu N. Asymmetrical relationships between the right and left heights of the sylvian end points in right- and left-pawed male and female cats: similarities with planum temporale asymmetries in human brain. Int J Neurosci. 1992;67(1–4):81–91.
Buchert R, Kluge A, Tossici-Bolt L, Dickson J, Bronzel M, Lange C, et al. Reduction in camera-specific variability in [123I]FP-CIT SPECT outcome measures by image reconstruction optimized for multisite settings: impact on age-dependence of the specific binding ratio in the ENC-DAT database of healthy controls. Eur J Nucl Med Mol Imaging. 2016;43(7):1323–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayashi, T., Mishina, M., Sakamaki, M. et al. Effect of brain atrophy in quantitative analysis of 123I-ioflupane SPECT. Ann Nucl Med 33, 579–585 (2019). https://doi.org/10.1007/s12149-019-01367-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01367-4