Abstract
Objective
Positron emission tomography in association with magnetic resonance imaging (PET/MR) and 68Ga-PSMA-11 has shown superior detection in recurrent prostate cancer patients as compared to PET/computed tomography (PET/CT). There are, however, several technological differences between PET/CT and PET/MR systems which affect the PET image quality. The objective of this study was to assess the reproducibility of PET/CT and PET/MR SUV’s in recurrent prostate cancer patients. We randomized the patients regarding the order of the PET/CT and PET/MR scans to reduce the influence of tracer uptake as a function of time.
Methods
Thirty patients, all with biochemical recurrence after radical prostatectomy, underwent whole-body PET/CT and PET/MR scans after intravenous injection of a single dose of 68Ga-PSMA-11. Fifteen patients underwent PET/CT first and 15 patients underwent PET/MR first. Volumes of interest on tumor lesions were outlined and maximum standardized uptake value (SUVmax) corrected for lean body mass was calculated. Correlation and agreement between scans were assessed by generalized linear mixed-effects models and Bland–Altman analysis. The association between SUV, patient characteristics and imaging parameters was assessed.
Results
Eighteen of the 30 evaluated patients had at least one positive lesion, giving an overall detection rate of 60%. In total, there were 34 visible lesions: 5 local recurrences, 22 lymph node metastases and 7 bone metastases. One group acquired PET/CT and PET/MR at median time points of 63.0 and 159.0 min, while the other group acquired PET/MR and PET/CT at median time points of 92.0 and 149.0 min. SUVmax between scans was linearly correlated, described by the equation Y(PET/CT SUVmax) = 0.75 + 1.00 × (PET/MR SUVmax), on average 20% higher on PET/CT than on PET/MR. SUV associated significantly only with type of lesion, scan time post-injection and acquisition time per bed position.
Conclusions
SUVmax from PET/CT and PET/MR are linearly correlated, on average 20% higher on PET/CT than on PET/MR and should, therefore, not be used interchangeably in patient follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate-specific membrane antigen (PSMA) is a transmembrane protein overexpressed in most prostate cancer cells [1]. Since the development of the PSMA inhibitor ligand Glu-urea-Lys(Ahx)-HBED-CC (PSMA-11) labeled with 68Ga, many studies have reported its use as a highly specific positron emission tomography (PET) imaging probe for detection and staging of prostate cancers [2]. Due to its high detection rate of lesions even at low serum prostate-specific antigen (PSA) levels, 68Ga-PSMA-11 PET combined with computed tomography (PET/CT) has become an important tool to confirm biochemical recurrence of prostate cancer [3,4,5,6,7].
PET in association with magnetic resonance imaging (PET/MR) combines high soft tissue contrast resolution of MR with metabolic information from PET, with the further advantage of low patient radiation exposure. Studies in recurrent prostate cancer patients have shown a superior detection of 68Ga-PSMA-11 PET/MR compared with PET/CT, but also pointed to limitations of the technique, such as the appearance of halo artifact around the urinary bladder, with underestimated tracer uptake and limited lesion visualization [8,9,10,11]. There are several technological differences between PET/CT and PET/MR systems, as the composition of the PET detectors and methods used for attenuation and scatter correction, which in turn affect the PET image quality [12]. Correction for tissue attenuation of photons is essential to provide accurate and quantitative PET images. In PET/CT systems, a low-dose CT scan provides pixel-wise image attenuation coefficients, which are linearly transformed in a PET 511 keV attenuation map and used for correction. MR images, on the other hand, measure proton density instead of electron density and do not provide a straightforward way of estimating photon attenuation. The most common method to transform the MR image into a CT-like attenuation map is by segmentation, which divides the image into air, fat, lung, and soft tissue and sets linear attenuation coefficients per segment [13]. Bone is usually included in the soft tissue compartment, which leads to underestimation of the bone uptake due to lower attenuation coefficients [14].
Semi-quantitative measures of PET tracer uptake serve as an important tool to complement visual analysis in routine nuclear medicine practice for treatment monitoring [15]. The standardized uptake value (SUV), which is tracer uptake in a region of interest, normalized to injected activity and body mass, is the most commonly employed measure, due to its simple calculation. The most frequently used SUV metric is SUVmax, which is a single-voxel value with the maximum SUV within a volume. SUVmax is straightforward, operator-independent and is considered the de facto standard due to its widespread use.
Several groups have studied the impact of MR-based attenuation correction on PET quantification. Kim et al. evaluated the SUV bias using segmented-based attenuation maps from the PET/CT data and showed that PET/MR SUV’s are underestimated in bone and liver lesions [16]. For 68Ga-PSMA-11 PET, a few studies have compared SUV’s between same-day sequential PET/CT and PET/MR scans. The majority of these studies acquired PET/CT first at 1 h post-injection (p.i.) and PET/MR at 3 h p.i., reporting higher SUVmax from PET/MR compared to PET/CT [8, 10]. Another group acquired PET/MR first at 35 min p.i., followed by PET/CT at 70 min p.i [17]. The authors reported significantly higher values in the late PET/CT scan in comparison to the early PET/MR scans. Although 68Ga-PSMA-11 has a rapid uptake in tumor tissue, it continues to accumulate during 60 min-long dynamic PET acquisitions [18, 19]. Also late imaging, up to 3 h p.i., has shown increasing SUV’s in lesions over time [20]. Due to the difference in tracer uptake time between the PET/CT and PET/MR scans, the studies were not able to conclude what caused the SUV bias: different scan time post-injection or different imaging modalities.
Here, we designed the study to reduce the influence of tracer uptake as a function of time by randomizing the patients regarding the order of the PET/CT and PET/MR scans. The objective was to assess the relationship and agreement between PET/CT and PET/MR SUV’s in recurrent prostate cancer patients and to propose an equation that describes the relationship between SUV’s from the two imaging modalities. We also assessed the association between SUV, patient characteristics and imaging parameters.
Materials and methods
Patients
This observational prospective study was approved by the Ethics Committee at our institution and conducted in accordance with the Helsinki Declaration. Thirty patients (mean age 67.8 ± 6.9), all with biochemical recurrence with elevated PSA levels after radical prostatectomy, were invited to participate in the study. Fifteen patients underwent whole-body PET/CT first, followed by PET/MR and 15 patients underwent whole-body PET/MR first, followed by PET/CT. They received an intravenous injection of a single dose of 68Ga-PSMA-HBED-CC (median injected activity 194.25 MBq; mean injected activity per body weight 2.31 MBq/kg). All subjects gave informed written consent to participate in the study.
PET/CT protocol
Whole-body PET/CT data were acquired on a Biograph mCT scanner with time-of-flight (32-slice CT; Siemens Medical Solutions, Erlangen, Germany), with arms up from skull base to mid-thigh, with 4.0 min of median acquisition per bed position (range 4–8 min; time per bed position varying according to scan time p.i.). The PET images were reconstructed using the Ultra-HD method (TrueX + time-of-flight) with 2 iterations, 21 subsets, 200 × 200 matrix and 3 mm Gaussian filter with CT-based attenuation correction (100 kV, 156 mAs, pitch 1.5, 0.5 s/rotation).
PET/MR protocol
Whole-body PET/MR data were acquired on a Biograph mMR scanner (Siemens Medical Solutions, Erlangen, Germany), with arms down from skull base to mid-thigh, with 6.0 min of median acquisition per bed position (range 4–8 min; time per bed position varying according to scan time p.i.). We used a 24-channel spine RF coil integrated within the MR bed and 3 surface body coils (6 channels each) to cover the thorax, abdomen and pelvis. For the neck we used an 8-channel radio-frequency (RF) head/neck coil.
Simultaneously to PET acquisition, the following MR data were acquired: axial T1 volumetric interpolated breath-hold examination (VIBE) Dixon (18 s), coronal T2 half-fourier single shot turbo spin echo (HASTE) without fat suppression with breath holding (23 s) and coronal short-TI inversion recovery (STIR) without breath holding.
The PET images were reconstructed with a 3D-OSEM algorithm (3 iteration, 21 subsets, matrix 256 × 256, 4 mm Gaussian filter) and corrected for attenuation using Dixon-based MR sequences.
Image analysis
We used the software syngo.via (Siemens Medical Solutions, Erlangen, Germany) for image analysis. The PET/CT and PET/MR images were reviewed by a board-certified nuclear medicine physician and a radiologist. Focal 68Ga-PSMA-11 uptake on the PET/CT images with corresponding changes on the CT scan, were considered positive lesions and were then confirmed on the PET/MR images. The lesions were identified as either local recurrence, lymph node or bone metastases. Volumes of interests (VOI’s) were outlined on the PET/CT and PET/MR images by a single technologist and reviewed by a nuclear medicine physician and a radiologist.
SUVmax was measured as a single-voxel value with the maximum SUV within the VOI and calculated according to the following formula, normalized to lean body mass (LBM) [21]:
where LBM = 9270 × weight (kg)/(6680 + 216 × BMI (kg/m2)) for males. BMI is body mass index (weight/height2).
Statistical analysis
Data were analyzed using software R [version 3.4.1, R Core Team (2017)] with packages lme4, MuMIn e BlandAltmanLeh. All distributions were visually checked and tested for normality and described accordingly by mean ± standard deviation (SD) or by median values with 25th and 75th percentiles (Q1 and Q3). Differences in patient characteristics and imaging parameters between the two groups were assessed using either Student’s t test or Mann–Whitney U test, depending on normal distribution. The level of significance was set to 5%.
Distribution and correlation of PET/CT and PET/MR SUV’s were visually assessed through box and scatter plots. Agreement between scans was measured through Bland–Altman plots and metrics [mean percentage differences between SUV’s with 95% limits of agreement (LOA)].
For quantitative comparison of PET/CT and PET/MR data, we used generalized linear mixed-effects models due to dependency between multiple lesions from the same patient. The following linear model was fitted to the data: Y(PET/CT SUVmax) = a + b × (PET/MR SUVmax), where a is the intercept and b is the slope. We assumed that if a = 0 and b = 1, then there is evidence that PET/CT and PET/MR are similar. For model fitting, we used patient as random effect, gamma distribution and identity link function. To determine the proportion of variation explained by the model, we calculated the coefficient of determination R2 using the method by Nagelkerke [22].
To determine the association between PET/CT and PET/MR SUVmax, type of lesion, age, BMI, injected activity, PSA, scan time post-injection and acquisition time per bed position, we fitted a generalized linear mixed-effects model. We fitted data to the following general equation: ln(PET/CT SUVmax) = µ + β1 × v1 + β2 × v2 + …, where ln is the natural logarithm, µ is the intercept, β are the effects and v are the parameters tested in the model. For the model, we assumed patient as random effect, gamma distribution and logarithmic link function. Significance level was set to 5%.
Results
The median time span from radical prostatectomy surgery to biochemical recurrence was 50 months (range 2–147 months) with a median PSA level of 0.70 ng/mL (range 0.12–17.28 ng/mL).
Eighteen of the 30 evaluated patients had at least one positive lesion, giving an overall detection rate of 60%. For PSA values below 1 ng/mL, the detection rate was 53% (10 of 19 patients) and for PSA values of 1 ng/mL and above, the detection rate was 73% (8 of 11 patients). For PSA above 1.5 ng/mL, the detection rate was 100% (6 patients). In total, there were 34 visible lesions, ranging from 1 to 7 per patient, of which 5 were identified as local recurrence (14.7%), 22 as lymph node metastases (64.7%) and 7 as bone metastases (20.6%). Both PET/CT and PET/MR systems generated images with diagnostic image quality and all lesion detected on the PET/CT scan were confirmed on the PET/MR scan (Fig. 1).
Patient with biochemical recurrence presenting with a metastasis in a left internal iliac lymph node. Both PET/CT (a, c, e 160 min post-injection) and PET/MR (b, d, f 110 min post-injection) show the same lymph node with intense, abnormal 68Ga-PSMA-11 uptake: SUVmax 48.26 on PET/CT and 30.95 on PET/MR
The two patient groups showed no significant differences regarding age, BMI, injected activity per patient weight or PSA level (Table 1). The total number of bone lesions was small and distributed unevenly between groups (1 versus 6). Acquisition time per bed position showed a small absolute, but statistically significant, difference between groups (p < 0.001 for PET/CT scan and p = 0.002 for PET/MR scan). The group of patients, which acquired PET/CT first had the PET/CT scan at a median time point of 63.0 min (Q1 = 56.5, Q3 = 69.0) and the PET/MR scan at a median time point of 159.0 min (Q1 = 148.5, Q3 = 170.0) p.i. The patient group, which acquired PET/MR first, underwent PET/MR at a median time point of 92.0 min (Q1 = 85.5, Q3 = 98.0) and the PET/CT at a median time point of 149.0 min (Q1 = 138.0, Q3 = 156.0) p.i.
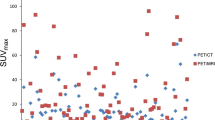
SUVmax exhibited non-normal distributions and quite large variability, with median values close to the lower quartile and two outliers located at the high end (Fig. 2). A visual examination of the box plot in Fig. 2 shows that the PET/CT SUV’s were slightly higher than the PET/MR SUV’s, although the median SUV’s from the two scans were similar: 4.14 for PET/CT and 4.18 for PET/MR, respectively.
Figure 3 displays the scatter plot with the line of identity and the mixed-model regression line. The mixed-model estimated the slope and intercept with corresponding 95% confidence intervals to 1.00 (0.73, 1.27) and 0.75 (− 0.10, 1.61), giving the following relationship: Y(PET/CT SUVmax) = 0.75 + 1.00 × (PET/MR SUVmax). The confidence intervals included 1 for the slope and 0 for the intercept, which demonstrates that PET/CT and PET/MR SUVmax were statistically similar. The coefficient of determination R2 for the regression model was 0.77.
Scatter and Bland–Altman plots of SUVmax comparing PET/CT and PET/MR scans. The scatter plot indicates the line of equality (dashed) and model regression line (solid) with equation and corresponding 95% confidence interval (grey area). The Bland–Altman plot indicates the mean percentage difference (solid line) between PET/CT and PET/MR scans and upper and lower 95% limits of agreement (dashed lines)
Bland–Altman analysis estimated a mean percentage difference between PET/CT and PET/MR SUVmax of 18.72% (Fig. 3). SUVmax showed a wide range of 95% LOA’s, ranging from − 116.58 to + 154.03%, with the largest percentage differences for small SUV’s. Local recurrence lesions showed a mean percentage difference of 4.76 (LOA’s − 188.89 to 198.44), lymph nodes a mean percentage difference of 22.15 (LOA’s − 99.72 to 144.03) and bone lesions a mean percentage difference of 17.90 (LOA’s − 133.45 to 169.25).
Generalized linear mixed-effects models of PET/CT and PET/MR SUV, patient characteristics and imaging parameters showed a significant association only between the two SUV’s (p < 0.001), type of lesion (p = 0.019), scan time post-injection (p = 0.003) and acquisition time per bed position (p = 0.021) (Table 2). The variables patient age (p = 0.257), BMI (p = 0.118), PSA (p = 0.510) and injected activity (p = 0.053) did not associate significantly with SUV. The final predictive model for PET/CT SUV resulted in the following equation: ln(PET/CT SUVmax) = ln(1.20) + ln(1.14) × (PET/MR SUVmax) + ln(0.63) × (bone lesion) + ln × (1.16) × (local recurrence) + ln(0.98) × (PET/MR scan time) + ln(1.45) × (PET/MR time per bed position). The final model resulted in a coefficient of determination R2 of 0.830.
Discussion
Despite the increasing number of institutions using PET/MR clinically, the quantitative reproducibility of PET/MR and PET/CT metrics is still not clear. We show here that SUVmax between same-day randomized 68Ga-PSMA-11 PET/CT and PET/MR scans in recurrent prostate cancer patients agree well and correlate linearly, but present a mean percentage difference of almost 20%. The relationship can be described by the linear equation Y(PET/CT SUVmax) = 0.75 + 1.00 × (PET/MR SUVmax), close to identity. This strong correlation is in accordance with previous studies [17, 23] and the fact that the 95% confidence interval included 1 for the slope and 0 for the intercept, assures us that the similarity between scans is significant.
To exemplify the relationship between the two imaging modalities, a PET/MR SUV of 2.00 would result in a PET/CT SUV of 2.75, which is a 37% difference and a PET/MR SUV of 5.00 would result in a PET/CT of 5.75, which is a 15% difference. This large variation at small SUV’s was also observed in the Bland–Altman analysis, where variations of 150% could be observed for small SUV’s, while for SUV’s above 5, the variation stabilized at around 20%. Bone lesions did not show larger difference between scans than other lesions, which is surprising as the bias due to attenuation correction is usually larger in bone tissue. The number of bone lesions between groups was quite different: 6 in the group which had the PET/CT scan first and one bone in the group which had the PET/MR scan first. However, the mean difference of SUV of the bone lesions was very similar to the mean difference when considering all lesions (17.9 versus 18.7%). We, therefore, believe that this uneven distribution of bone lesions between groups did not affect the overall result.
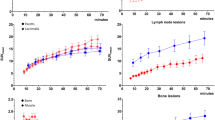
Our results are in line with Groshar et al. [24], who assessed the reproducibility of randomized same-day PET/CT and PET/MR SUV’s in primary lesions using 18F-fluorodeoxyglucose (FDG). They demonstrated a strong correlation between PET/CT and PET/MR SUV’s, described by the linear equation Y(PET/CT SUV) = 0.8 + 1.01 × (PET/MR SUV), almost identical to the equation we reported. The Bland–Altman analysis gave a mean difference between 18F-FDG PET/CT and PET/MR SUV’s of 13.8%, with 95% LOA’s between − 27.0 and 54.6%. The total number of lesions (n = 63) as compared to our study (n = 34) might have influenced the smaller variation observed in their study. Also the fact that they used SUVpeak, which is the average SUV within a volume of 1 mL around the voxel of SUVmax, instead of the single-voxel SUVmax might have contributed to the smaller variation. In fact the PERCIST criteria recommends SUVpeak for being less subject to variance than SUVmax [25]. Our study population of recurrent prostate cancer patients had small lesions, majority less than 1 mL (data not shown), which excluded the possibility of calculating SUVpeak. Besides, a recently published guideline recommends SUVmax for quantification of 68Ga-PSMA PET/CT images [26].
Regarding SUV reproducibility, there are conflicting reports in the literature. Afshar-Oromieh et al. acquired 68Ga-PSMA PET/CT first followed by PET/MR and reported higher SUVmax in the late PET/MR scan in 55 lesions, lower SUVmax in the late PET/MR in 7 lesions and no SUV change between scans in 7 lesions [8]. Lütje et al. reported significantly higher SUVmax on the late 68Ga-PSMA PET/MR scan compared to the early PET/CT scan (17.6 vs 15.1, p = 0.0019) [10]. On the other hand, Domachevsky et al. acquired PET/MR first followed by PET/CT and reported SUV’s on average 30% higher on the late PET/CT scan in comparison to the early PET/MR scan with LOA’s between − 13.4 and 73.4% [17]. In our study half of the patients underwent PET/CT first and the other half PET/MR first, thereby reducing the uptake time bias. We showed that SUVmax are on average 20% higher on PET/CT in comparison with PET/MR. Interestingly, our PET/CT data were actually acquired slightly before the PET/MR data (63 and 149 min p.i. for PET/CT vs. 92 and 159 min p.i. for PET/MR). Therefore, the higher PET/CT SUV cannot be explained by longer accumulation of the 68Ga-PSMA in the PET/CT scan, but rather by the inherent differences of the imaging modalities. Actually, if the PET/MR scan had been acquired 30 min earlier at the same median time point as the PET/CT, probably the underestimation of PET/MR SUV in comparison to PET/CT SUV would have been even larger than 20%.
The use of dedicated phantoms has an important role for calibration of scanners and validation of new imaging modalities. However, the use of phantoms for PET/MR systems is not as straightforward as for PET/CT systems. In patient imaging, the MR image is segmented into three (air, lung, and soft tissue) or four compartments (air, fat, lung, and soft tissue) depending on the scanner model. This method is not suitable for water-filled plastic phantoms, having different densities than human tissue. Boellard et al. compared clinical MR-attenuation correction protocols and a proposed dedicated phantom protocol, and showed that the clinical protocol, which segmented the MR image into three or four compartments, underestimated the radioactivity concentration up to 27%, while a phantom protocol, which segmented the MR image into only two compartments (water and air) reduced the bias to 8% [27]. Keller et al. reported on average 16% underestimated radioactivity concentration on PET/MR in comparison to PET/CT, when using MR-based attenuation maps [28]. In our study, we acquired the PET/CT and PET/MR data on Siemens Biograph mCT an mMR systems, respectively. Karlberg et al. published recently a quantitative comparison of the two systems and concluded that the mMR image quality was close to that of the mCT and that the differences between the systems were mainly due to the time-of-flight PET detector of the mCT, which improved image quality at small uptake volumes [12]. The smallest volume tested in the study was a sphere of 10 mm in inner diameter, which is in line with the recurrent prostate cancer lesions in our study.
Although SUV is a semi-quantitative measure of tracer uptake, different from true quantitative kinetic modeling, it is an important tool to complement visual analysis in routine nuclear medicine practice for monitoring of disease progression and treatment response. In recurrent and metastatic prostate cancers, Einspieler et al. demonstrated a significant correlation between SUVmax and serum PSA levels and received therapy [7], while Verburg et al. were unable to demonstrate a significant relationship between the same parameters [29]. Similar to Einspieler et al., we did not observe a significant association between PSA and SUV, instead only lesion type, post-injection scan time and acquisition time per bed position associated significantly with SUV. The significant association between SUV and acquisition time per bed position should be a results of an indirect association with post-injection scan time, since for late scan times, time per bed position is increased to account for the reduced count statistics.
With the increasing use of 68Ga-PSMA-11 PET/MR in clinical settings, the reproducibility of SUVmax between PET/CT and PET/MR is essential. The linear mixed-model equation provides an average correction factor of SUVmax when comparing 68Ga-PSMA-11 PET data from the two imaging modalities. The coefficient of determination was 0.77, which means that 77% of the variation of PET/CT SUVmax was explained by the PET/MR SUVmax. The remaining variation could in part be due to image noise, but our data suggest that there is in fact a difference in SUVmax explained by inherent differences of the imaging modality, as detector type, reconstruction methods and attenuation correction. Although we performed our study on Siemens Biograph mCT and mMR scanners, the basic principles are common to all PET/CT and PET/MR systems and the result should, therefore, be useful for other scanner comparisons.
The observed overall detection rate of 60% (18 of 30 patients) was considerably lower than previous studies [3,4,5,6,7,8,9, 11], but similar to Uprimny et al., who reported a detection rate of 62.1% [30]. However, the small median PSA value of our patient group of 0.70 ng/mL (Q1 = 0.41, Q3 = 1.19), might explain the low detection rate. For the 6 patients who presented a PSA value above 1.5 ng/mL, all had PET positive lesions.
We identify some limitations of our study. The number of patients and number lesions were small, but despite the small numbers, there was enough power to obtain significance in the mixed-model analysis. The randomization resulted in equal number of patients in each group (n = 15 in each group), but the number of lesions (n = 20 and n = 14) and the type of lesions were not the same between groups. Different post-injection scan time for the first scan (PET/CT at 63 min vs PET/MR at 92 min) might have diminished slightly the real difference between scans. The study also lacks histopathological confirmation of the 68Ga-PSMA-11 positive lesions.
Conclusion
Here we show that SUVmax from randomized same-day PET/CT and PET/MR scans in recurrent prostate cancer patients, are linearly correlated, but on average 20% higher on PET/CT than on PET/MR. Caution is necessary when comparing SUV’s from 68Ga-PSMA-11 PET/CT and PET/MR scans, particularly in low-uptake lesions, and should, therefore, not be used interchangeably in patient follow-up.
References
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–5.
Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–35.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42(8):1284–94.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of Hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–74.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2):197–209.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–68.
Einspieler I, Rauscher I, Duwel C, Kronke M, Rischpler C, Habl G, et al. Detection efficacy of hybrid (68)Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by phoenix criteria. J Nucl Med. 2017;58(7):1081–7.
Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68 Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014;41(5):887–97.
Freitag MT, Radtke JP, Afshar-Oromieh A, Roethke MC, Hadaschik BA, Gleave M, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in (68)Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging. 2017;44(5):776–87.
Lutje S, Cohnen J, Gomez B, Gruneisen J, Sawicki L, Rubben H, et al. Integrated (68)Ga-HBED-CC-PSMA-PET/MRI in patients with suspected recurrent prostate cancer. Nuklearmedizin. 2017;56(3):73–81.
Kranzbuhler B, Nagel H, Becker AS, Muller J, Huellner M, Stolzmann P, et al. Clinical performance of (68)Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2018;45(1):20–30.
Karlberg AM, Saether O, Eikenes L, Goa PE. Quantitative comparison of PET performance-Siemens Biograph mCT and mMR. EJNMMI Phys. 2016;3(1):5.
Schulz V, Torres-Espallardo I, Renisch S, Hu Z, Ojha N, Bornert P, et al. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging. 2011;38(1):138–52.
Paulus DH, Quick HH, Geppert C, Fenchel M, Zhan Y, Hermosillo G, et al. Whole-body PET/MR imaging: quantitative evaluation of a novel model-based mr attenuation correction method including bone. J Nucl Med. 2015;56(7):1061–6.
Tomasi G, Turkheimer F, Aboagye E. Importance of quantification for the analysis of PET data in oncology: review of current methods and trends for the future. Mol Imaging Biol. 2012;14(2):131–46.
Kim JH, Lee JS, Song IC, Lee DS. Comparison of segmentation-based attenuation correction methods for PET/MRI: evaluation of bone and liver standardized uptake value with oncologic PET/CT data. J Nucl Med. 2012;53(12):1878–82.
Domachevsky L, Bernstine H, Goldberg N, Nidam M, Stern D, Sosna J, et al. Early (68)GA-PSMA PET/MRI acquisition: assessment of lesion detectability and PET metrics in patients with prostate cancer undergoing same-day late PET/CT. Clin Radiol. 2017;72(11):944–50.
Sachpekidis C, Eder M, Kopka K, Mier W, Hadaschik BA, Haberkorn U, et al. (68)Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(7):1288–99.
Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68 Ga-PSMA-11 dynamic PET/CT imaging in primary prostate cancer. Clin Nucl Med. 2016;41(11):e473–9.
Pfob CH, Ziegler S, Graner FP, Kohner M, Schachoff S, Blechert B, et al. Biodistribution and radiation dosimetry of (68)Ga-PSMA HBED CC-a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(11):1962–70.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–2.
Freitag MT, Radtke JP, Hadaschik BA, Kopp-Schneider A, Eder M, Kopka K, et al. Comparison of hybrid (68)Ga-PSMA PET/MRI and (68)Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(1):70–83.
Groshar D, Bernstine H, Goldberg N, Nidam M, Stein D, Abadi-Korek I, et al. Reproducibility and repeatability of same-day two sequential FDG PET/MR and PET/CT. Cancer Imaging. 2017;17(1):11.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014–24.
Boellaard R, Rausch I, Beyer T, Delso G, Yaqub M, Quick HH, et al. Quality control for quantitative multicenter whole-body PET/MR studies: a NEMA image quality phantom study with three current PET/MR systems. Med Phys. 2015;42(10):5961–9.
Keller SH, Jakoby B, Svalling S, Kjaer A, Hojgaard L, Klausen TL. Cross-calibration of the Siemens mMR: easily acquired accurate PET phantom measurements, long-term stability and reproducibility. EJNMMI Phys. 2016;3(1):11.
Verburg FA, Pfister D, Drude NI, Mottaghy FM, Behrendt FF. PSA levels, PSA doubling time, Gleason score and prior therapy cannot predict measured uptake of [68Ga]PSMA-HBED-CC lesion uptake in recurrent/metastatic prostate cancer. Nuklearmedizin. 2017;56(6):225–32.
Uprimny C, Kroiss AS, Fritz J, Decristoforo C, Kendler D, von Guggenberg E, et al. Early PET imaging with [68]Ga-PSMA-11 increases the detection rate of local recurrence in prostate cancer patients with biochemical recurrence. Eur J Nucl Med Mol Imaging. 2017;44(10):1647–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ringheim, A., Campos Neto, G.d., Martins, K.M. et al. Reproducibility of standardized uptake values of same-day randomized 68Ga-PSMA-11 PET/CT and PET/MR scans in recurrent prostate cancer patients. Ann Nucl Med 32, 523–531 (2018). https://doi.org/10.1007/s12149-018-1275-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1275-7