Abstract
Objective
One of the most interesting clinical applications of 18F-FDG PET imaging in neurodegenerative pathologies is that of establishing the prognosis of patients with mild cognitive impairment (MCI), some of whom have a high risk of progressing to Alzheimer’s disease (AD). One method of analyzing these images is to perform statistical parametric mapping (SPM) analysis. Spatial normalization is a critical step in such an analysis. The purpose of this study was to assess the effect of using different methods of spatial normalization on the results of SPM analysis of 18F-FDG PET images by comparing patients with MCI and controls.
Methods
We evaluated the results of three spatial normalization methods in an SPM analysis by comparing patients diagnosed with MCI with a group of control subjects. We tested three methods of spatial normalization: MRI-DARTEL and MRI-SPM8, which combine structural and functional images, and FDG-SPM8, which is based on the functional images only.
Results
The results obtained with the three methods were consistent in terms of the main pattern of functional alterations detected; namely, a bilateral reduction in glucose metabolism in the frontal and parietal cortices in the patient group. However, MRI-SPM8 also revealed differences in the left temporal cortex, and MRI-DARTEL revealed further differences in the left temporal cortex, precuneus, and left posterior cingulate.
Conclusions
The results obtained with MRI-DARTEL were the most consistent with the pattern of changes in AD. When we compared our observations with those of previous reports, MRI-SPM8 and FDG-SPM8 seemed to show an incomplete pattern. Our results suggest that basing the spatial normalization method on functional images only can considerably impair the results of SPM analysis of 18F-FDG PET studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A potential clinical application of 18F-FDG PET imaging in neurodegenerative pathologies is prediction of the prognosis of patients with mild cognitive impairment (MCI). An abnormal pattern of brain metabolism in MCI indicates a higher risk of developing dementia within the following 2 years (sensitivity 84 % and specificity 93 %) [1]. When compared with controls, patients with MCI who subsequently progressed to Alzheimer’s disease (AD) showed a pattern of metabolic alterations consistent with those observed in AD patients [2, 3].
PET images can be quantified and analyzed by focusing on specific regions of interest (ROI) a priori or by following a whole-brain approach based on the voxel-wise methods that do not require a priori hypotheses about the localization of metabolic changes. Statistical parametric mapping (SPM) (http://www.fil.ion.ucl.ac.uk/spm, Wellcome Department of Imaging Neuroscience, Institute of Neurology, UCL, London, UK) [4] is one of the most widely used software packages for voxel-wise analysis of functional images such as PET images. Spatial normalization of individual PET images to an anatomical reference template is a critical step in SPM. When images are compared between subjects, spatial normalization is usually achieved by matching a template through non-linear transformations of each brain.
Most non-linear deformation algorithms try to minimize differences in brain morphology that cannot be accounted for by linear transformations [5]. Although these transformations can be obtained from 18F-FDG PET images, more reliability can be expected using better anatomical information from structural images. In this case, the deformation parameters, commonly obtained from structural T1 images, are applied to the corresponding PET images, which are previously co-registered with the MRI scan. Spatial normalization based on the structural images is allegedly more accurate than that based on the functional images, as structural images have higher spatial resolution and convey much better anatomical information [6].
SPM recently incorporated a newer method for image registration based on the diffeomorphic algorithms; namely, DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) [7], which is more geometrically accurate than the previous algorithms and could increase the sensitivity of voxel-wise analyses [8]. However, DARTEL is not yet widely used for the spatial normalization of 18F-FDG PET images.
We performed a study to investigate the effect of using different spatial normalization procedures on the outcome of SPM analysis of 18F-FDG PET images and, in particular, to assess the potential benefit of using DARTEL for spatial normalization. We evaluated the results of three spatial normalization methods in an SPM analysis by comparing patients diagnosed with MCI with a group of control subjects. The quality of the results was interpreted by comparison with the well-known pattern of cerebral hypometabolism characteristic of AD patients described in the literature [1], [9], [10], [11], [12], [2]. Because MCI may indicate the onset of AD, a robust and accurate method of identifying susceptible patients could prove useful in clinical practice [13].
Materials and methods
Patients and control
We studied a total of 31 subjects (20 patients and 11 controls) who were recruited at the Behavioral Neurology Unit of Hospital General Universitario Gregorio Marañón in Madrid, Spain. The control group included relatives and caregivers. All participants signed an informed consent, and the local ethics committee approved the study.
Both patients and controls underwent neurological assessments at the Behavioral Neurology Unit. Mental status was evaluated using Folstein’s ‘mini-mental’ test (MMSE) [14]. Neuropsychological testing was completed using a battery of tests to characterize cognitive deficits and their severity [15], [16], [17], [18], [19], [20].
The patients (mean age, 70 ± 7.7 years; 10 women) were all diagnosed with MCI using the criteria of [21], which are an extension of the criteria of [22]. The MCI criteria included self- and/or informant-based reporting of impairment in any cognitive function with preserved basic activities and no or minimal impairment in complex instrumental functions. Cognitive impairment had to be supported by an abnormal performance (1–1.5 SD below the expected performance according to age and education) in one or more tests from the neuropsychological examination described above. Patients belonged to the single domain amnestic category and had a clinical dementia rating (CDR) score of 0.5 [23]. However, they did not meet the criteria for dementia of DSM-IV-TR (the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision).
The 11 controls (mean age, 69 ± 7.8 years; 6 women) had no cognitive disorders or clinically relevant conditions or other circumstances that could interfere with cognitive performance. To be included in the study, they had to score above 24 in the adapted Spanish version of the MMSE (Table 1) [14, 24].
A functional 18F-FDG PET image and a structural T1-weighted MRI image were obtained for each subject according to the protocols described below.
Imaging protocol
18F-FDG PET images were obtained using a PET/CT GEMINI system (Philips Medical Systems) 30 min after the injection of 370 MBq of 18F-FDG with the subject at rest and eyes open in a dark room. The matrix size was 128 × 128 × 90 pixels and the voxel size 2 × 2 × 2 mm.
MRI data were acquired on a Philips Intera 1.5T MR scanner (Philips Medical Systems, Best, The Netherlands). The imaging protocol consisted of a volumetric T1-weighted 3D gradient echo sequence (flip angle = 30º; TR = 16 ms, TE = 4.6 ms; matrix size = 256 × 256; voxel size = 1 × 1 × 1.5; FOV = 256 mm).
Image processing
Before the statistical analysis, images were processed in four steps: (1) co-registration of FDG PET images of each subject to their corresponding T1-weighted MRI using mutual information algorithms available in SPM8 and an affine transformation, (2) spatial normalization using the three different approaches under comparison (see details below), (3) smoothing, and (4) global normalization.
Spatial normalization
We compared three methods of spatial normalization: the standard method for PET (FDG-SPM8), based only on information from the functional images, and two methods that make use of structural MRI data to estimate the normalization parameters (MRI-SPM8 and MRI-DARTEL). All the processing steps were implemented using routines from the SPM8 software package.
FDG-SPM8 has long been the standard procedure for analyzing PET scans using voxel-wise statistical analysis [8]. This normalization obtains the spatial transformation required for each PET image to match a PET template in MNI space. In this study, we used a specific template for FDG (Fig. 1) instead of the H2O template distributed with SPM, to avoid biased results. The construction of the template is described in Gispert et al. [6]. First, 17 FDG PET scans of control subjects were normalized to the standard 15O-H2O PET template provided with the SPM software application using the algorithm included in SPM99. Then, the FDG PET template was normalized by averaging these images and applying a smoothing Gaussian filter (FWHM = 8 × 8 × 8 mm) [6].
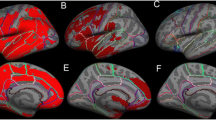
Spatial normalization methods used in the study. MRI-DARTEL normalization of MRI based on a study-specific template generated using the DARTEL algorithm; normalizations were later applied to co-registered PET images. MRI-SMP8 normalization of MRI based on the MNI-152 template and a standard SPM8 algorithm; normalizations were later applied to co-registered PET images. FDG-SPM8 normalization of PET based on the FDG-PET template and a standard SPM8 algorithm
The MRI-SPM8 procedure incorporates anatomical information from the subject’s MRI to achieve better normalization than using only functional (PET) data. This method computes the spatial non-linear transformation required to normalize the T1 images of each subject to the MNI-152 template using the “Normalize” function of SPM8. This template is included in SPM8. The transformation parameters obtained are then saved and applied to each co-registered PET image (Fig. 1).
MRI-DARTEL
MRI-DARTEL incorporates anatomical information from the MRI and applies the DARTEL tools in SPM8 to create a study-specific MRI template from MRI images of our 31 study subjects. The template is then normalized to the MNI space. This procedure of spatial normalization consists of four steps: (1) tissue segmentation of the T1 images for each subject using the new segmentation tool of SPM8; (2) construction of a study-specific template of each tissue type in MNI space; (3) estimation of the non-linear transformation required to warp grey and white matter images to match the study-specific template; (4) the transformation parameters obtained are then applied to each co-registered PET (Fig. 1).
Smoothing
We explored different Gaussian smoothing kernel sizes (6, 8, 10, and 12 mm, data not shown in the manuscript). As all four sizes yield a similar outcome, we chose the 8 × 8 × 8 mm FWHM filter, which is the most frequently used in other studies performed on images with a similar resolution.
Global normalization
Global cerebral metabolism varies greatly between subjects owing to the experimental conditions of each acquisition. Therefore, it is necessary to normalize the PET activity values of the images to enable intersubject comparisons. We used proportional scaling, as this method is considered the most suitable for intersubject PET imaging [25]. One step of this method is the creation of a mask. We used the option provided by the SPM8 software to create it. This mask contains those voxels with a value greater than the grand mean of the image divided by 8.
Statistical analysis
Following the four processing steps described, we generated three different data sets, one for each normalization method. To assess the effect of the spatial normalization method, we conducted three separate SPM analyses, one for each data set. The SPM analysis was based on a general linear model (GLM) to perform a voxel-wise comparison of PET images from patients and controls. The results of Student’s t tests at each voxel were used to generate statistical parametric maps of group differences showing regions where MCI patients had reduced glucose metabolism as compared to the control group. To obtain comparable results for each method, we heuristically selected the specific threshold for statistical significance that would have been used in a study including only one of the three particular data sets. Following this rationale, threshold parameters were set at an extent of 50 voxels (more than 2 times the FWHM) and p value of p < 0.02 (uncorrected). No corrections for multiple comparisons were attempted, since the main purpose of the study was not to confirm or reject the hypothesis of group differences between patients and controls, but rather to investigate the various outcomes of the statistical analysis depending on the choice of spatial normalization method used.
Results
Tables 2, 3, and 4 show the results for each normalization method, and a summary table (Table 5) compares the results from the three methods. The tables show the existence of significant clusters with reduced glucose metabolism in MCI as compared to controls, indicating anatomical localization, size (k), Talairach coordinates of peak maxima, and t value. Figures 2, 3, and 4 illustrate maximum intensity maps and significant clusters superimposed on the template T1 image.
Results obtained with MRI-DARTEL. Maximum intensity projections and significant clusters superimposed on the template T1 image showing slices from z = −47.5 mm to z = 76 mm using a 6.5-mm increment. Color blobs show the regions where MCI patients had significant glucose hypometabolism (p < 0.02, uncorrected, extent threshold of 50 voxels [k = 50])
Results obtained with MRI-SPM8. Maximum intensity projections and significant clusters superimposed on the template T1 image showing slices from z = −47.5 mm to z = 76 mm using a 6.5-mm increment. Color blobs show the regions where MCI patients had significant glucose hypometabolism (p < 0.02, uncorrected, extent threshold of 50 voxels [k = 50])
Results obtained with FDG-SPM8. Maximum intensity projections and significant clusters superimposed on the template T1 image showing a slices from z = −47.5 mm to z = 76 mm using a 6.5-mm increment. Color blobs show the regions where MCI patients had significant glucose hypometabolism (p < 0.02, uncorrected, extent threshold of 50 voxels [k = 50])
FDG-SPM8
The MCI group showed a statistically significant reduction in glucose metabolism in the frontal and parietal cortex bilaterally (Tables 4, 5; Fig. 4). The highest t value and largest cluster appeared in the supramarginal gyrus of the left parietal lobe. The next clusters in terms of t value and size were much smaller and located in the right parietal lobe, in the frontal lobe bilaterally and in the right precuneus (Tables 4, 5; Fig. 4).
MRI-SPM8
MRI-SPM8 yielded the same regions as FDG-SPM8, but with additional significant clusters. When compared with controls, statistically significant reduced glucose metabolism was observed in the MCI group in both the frontal and parietal cortices bilaterally, and in the left temporal cortex (Tables 3, 5; Fig. 3). The most relevant finding was a large cluster in the left inferior temporal lobe extending to the fusiform gyrus (Tables 3, 5).
MRI-DARTEL
MRI-DARTEL detected statistically significant hypometabolism in the same regions as FDG-SPM8 and MRI-SPM8, as well as in the frontal, parietal, and precuneus cortices bilaterally, the temporal cortex, and the posterior cingulate of the left hemisphere (Tables 2, 5; Fig. 2). The largest clusters appeared in the parietal lobes; the highest t value was recorded for the right lobe. The next cluster in terms of size was in the right frontal lobe. In the limbic system, significant clusters were located in the left hemisphere, posterior cingulate gyrus, and left parahippocampal gyrus.
In summary, the three methods are consistent in revealing significant clusters in the frontal and parietal cortices bilaterally, but with differences in extension and bilateral involvement, depending on the method used. In the case of MRI-DARTEL, the extension of the clusters was similar in both hemispheres, thus suggesting more bilateral involvement. MRI-SPM8 revealed no significant clusters in the precuneus or cingulate, and the results could be interpreted as a predominantly left distribution of the changes. FDG-SPM8 revealed no significant clusters in the cingulate or temporal lobe. The cluster with the highest t value was located in the left parietal lobe (Table 5).
Discussion
Our results show that SPM analysis based on MRI-DARTEL normalization detects a larger number of regions with reduced glucose metabolism in the MCI group than in the controls (Tables 2, 3, 4, 5; Fig. 2). MRI-SPM8 did not reveal significant differences in metabolism in the precuneus or cingulate (Tables 3, 5; Fig. 3). FDG-SPM8 only revealed significant differences in the frontal and parietal cortices and in the right precuneus, but not in the left precuneus, cingulate, or temporal cortex (Tables 4, 5; Fig. 4). These results confirm that the choice of spatial normalization method strongly affects the results of the statistical analysis [26].
Given the discrepancies in the results obtained with the different registration algorithms studied, it is necessary to determine which of the three is best in the absence of a gold standard. Our hypothesis is that, as our clinical setting is similar to that of many previous studies, methods which yield results closer to the most usual pattern will be considered the gold standard for the study. This approach is supported by a large body of literature about functional alterations in MCI and AD. An FDG PET pattern characteristic of AD has also been reported to occur in 33 % of subjects with MCI [11]. Those patients with MCI, who were at a high risk of progression to AD showed an FDG PET pattern of alterations that was characteristic of AD when compared with a control group [2]. This characteristic pattern of FDG PET alterations in AD comprises hypometabolism in the temporoparietal association cortex, posterior cingulate, precuneus, and associative frontolateral cortex [1]. Moreover, to further support the feasibility of our choice of gold standard, we conducted another analysis of a subgroup of patients who were known to progress to AD in a follow-up of the cohort 2 years after the initial scans. Despite the lower sample size, which led to poorer p values, the results matched the initial ones (data not shown), thus confirming the validity of the gold standard selected for this study.
The results obtained with MRI-DARTEL better match this pattern of hypometabolism (Tables 2, 5; Fig. 2); when MRI-SPM8 or FDG-SPM8 is used, the pattern is incomplete.
The greater anatomical detail and better spatial resolution of MRI compared with PET functional images may account for the more accurate spatial normalization obtained when using templates based on the structural images [5]. Using anatomical information for spatial normalization (MRI-DARTEL and MRI-SPM8 in the present study) ensures independence between the registration process and the subsequent statistical analysis. This independence is not possible when functional images are used for both spatial normalization and statistical analysis, thus generating a problem of circularity, because the same data are used to calculate the template and perform the statistical analysis [27]. Moreover, in cases of pathological abnormalities of brain anatomy, intersubject registration based on the functional images is less accurate, resulting in increased noise, dispersion of voxel values, and, therefore, less power in the statistical analysis [6], [28].
Our study is limited by the small number of subjects analyzed, which prevented us from applying corrections for multiple comparisons. However, our objective was to compare the registration algorithms under the same conditions and in a clinical practice setting. This limitation in sample size could have been overcome using publicly available data sets, such as the ADNI (http://www.adni.loni.ucla.edu), which would have enabled more quantitative comparisons between the three spatial normalization methods. Nevertheless, we were interested in exploring the specific effect of normalization in our clinical setting and under realistic technical conditions for image acquisition, especially for patients with MCI. Our study is limited because we have to assume that the true pattern of changes in our patients followed the well-known pattern of cerebral hypometabolism characteristic of AD patients described in the literature. We consider that this is a reasonable hypothesis, which is further supported by the fact that the patterns of metabolic differences obtained in our study are consistent with those reported using larger samples.
The need for acquisition of 3D T1WI MRI data in addition to FDG-PET constitutes a potential practical shortcoming of the MRI-DARTEL method, but many clinical protocols include an MRI study, especially if PET imaging is also performed.
Conclusions
Our results show that the choice of spatial normalization method may considerably affect the outcome of SPM analyses of 18F-FDG PET studies. When only functional information was used for spatial normalization, we detected fewer regions with significant differences in glucose metabolism, suggesting lack of statistical power. Therefore, results from PET studies obtained without anatomical MRI images should be interpreted with caution, as they may underestimate pathological findings in patients. Our results indicate that spatial normalization using the DARTEL algorithm is the most statistically powerful method of the three tested; therefore, we recommend its application in 18F-FDG PET studies.
References
Herholz K. PET studies in dementia. Ann Nucl Med. 2003;17(2):79–89.
Salmon E, Lekeu F, Garraux G, Guillaume B, Magis D, Luxen A, et al. Metabolic correlates of clinical heterogeneity in questionable Alzheimer’s disease. Neurobiol Aging. 2008;29(12):1823–9.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–18.
Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC. Human brain function (Chapter 4, Section 3). San Diego: Academic Press; 1997.
Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapp. 1999;7(4):254–66.
Gispert JD, Pascau J, Reig S, Martínez-Lázaro R, Molina V, García-Barreno P, et al. Influence of the normalization template on the outcome of statistical parametric mapping of PET scans. NeuroImage. 2003;19(3):601–12.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113.
Ashburner J, Chun-Chuan C, Guillaume F, Henson R, Kiebel S, Kilner J, et al. SPM8 Manual2009.
Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80(Special_Issue_2):S160–7.
Matsuda H. Role of neuroimaging in Alzheimer’s disease, with emphasis on brain perfusion SPECT. J Nucl Med. 2007;48(8):1289–300.
Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49(3):390–8.
Silverman DHS, Mosconi L, Ercoli L, Chen W, Small GW. Positron emission tomography scans obtained for the evaluation of cognitive dysfunction. Semin Nucl Med. 2008;38(4):251–61.
Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–55.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000;55(11):1613–20.
Lezak MD, editor. Neuropsycological assesment. 3rd ed. Oxford: Oxford University Press; 1995.
Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test—second edition. Adult version. Manual. Psychological Corporation, San Antonio, TX; 2000.
Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch de Psychologie. 1941;28:286–340.
Le Osterrieth P. test de copie d’une figure complexe. Arch de Psychologie. 1944;30:206–356.
Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621–6.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8.
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72.
Blesa R, Pujol M, Aguilar M, et al. Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia 2001;39:1150–1157.
Acton PD, Friston KJ. Statistical parametric mapping in functional neuroimaging: beyond PET and fMRI activation studies. Eur J Nucl Med. 1998;25(7):663–7.
Ishii K, Willoch F, Minoshima S, Drzezga A, Ficaro EP, Cross DJ, et al. Statistical brain mapping of 18F-FDG PET in Alzheimer’s disease: validation of anatomic standardization for atrophied brains. J Nucl Med. 2001;42(4):548–57.
Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage. 2001;14(6):1454–62.
Reig S, Penedo M, Gispert JD, Pascau J, Sánchez-González J, García-Barreno P, et al. Impact of ventricular enlargement on the measurement of metabolic activity in spatially normalized PET. NeuroImage. 2007;35(2):748–58.
Acknowledgment
This study was supported by the following institutions: Ministerio de Ciencia e Innovación, AMIT Programa CENIT. M.E. Martino was supported by the Contrato Río Hortega (Instituto de Salud Carlos III, Ministerio de Economía y Competitividad).
Conflict of interest
None of the authors have conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martino, M.E., de Villoria, J.G., Lacalle-Aurioles, M. et al. Comparison of different methods of spatial normalization of FDG-PET brain images in the voxel-wise analysis of MCI patients and controls. Ann Nucl Med 27, 600–609 (2013). https://doi.org/10.1007/s12149-013-0723-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0723-7