Abstract
Objective
Lymphoscintigraphy is an effective method for detecting sentinel lymph nodes (SLNs). However, the rate and degree of SLN detection is not uniform. We quantified SLNs detected with lymphoscintigraphy, and investigated correlations with factors that may influence detection. We then attempted to predict SLN metastasis from lymph node counts, comparing the predictions to subsequent biopsy results.
Methods
We assessed lymph node counts in 100 breast cancer patients in whom a single SLN was detected with a fixed lymphoscintigraphy procedure. We examined correlations between the counts and factors known to influence lymphoscintigraphic SLN detection (age, body mass index, tumor size, and presence or absence of metastasis), and determined reference values (lymph node counts of 10.0, 19.4 and 53.0) which were used to predict SLN metastasis in 100 subsequent patients. The predictions were then compared with the SLN biopsy findings.
Results
SLN counts correlated strongly with the presence or absence of metastasis, with metastasis-positive lymph nodes showing significantly lower counts than negative nodes (p < 0.001). Prediction of SLN metastasis achieved a 100% positive predictive value at a reference value of 10.0, and a 100% negative predictive value at a reference value of 53.0. At a reference value of 19.4, the sensitivity, specificity, and diagnostic accuracy were 77.8, 73.2, and 74.0%, respectively.
Conclusions
The SLN counts detected with lymphoscintigraphy were significantly lower in metastasis-positive lymph nodes than in metastasis-negative lymph nodes. This suggests that prediction of SLN metastasis in breast cancer is possible using lymphoscintigraphy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence or absence of metastasis in the axillary lymph nodes in breast cancer is a key factor for deciding the therapeutic strategy [1]. The sentinel lymph node (SLN) reflects the metastatic status of the axillary lymph nodes [2–5]. The idea that the absence of SLN metastasis indicates absence of metastasis in other axillary lymph nodes is used to decide whether to omit axillary lymph node dissection [6, 7], reducing the area of excision and enhancing the patient’s quality of life. Diagnosis of SLN metastasis is made intraoperatively through biopsy. Thus, it has not been possible to determine preoperatively whether axillary lymph node dissection is necessary.
Lymphoscintigraphy is currently used preoperatively for SLN identification, though not for prediction of SLN metastasis. Previous studies have shown that SLN visualization in patients with breast cancer is influenced by several factors, including procedural variations [8–12] and such patient factors as age, body mass index (BMI), tumor size, and metastasis to the SLN [13–15]. While those studies have served to advance the SLN detection rate, the detection rates have not been uniform, and the degree of detection has not yet been assessed.
In this study, lymphoscintigraphy was conducted with a fixed procedure, and the degree of SLN detection was quantified by assessing lymph node counts. Correlation of the lymph node counts with patient factors showed that the counts correlated most strongly with the presence or absence of metastasis. On that basis, reference values derived from the lymph node counts were used to attempt prediction of SLN metastasis. Finally, the predictions of metastasis were verified by comparing them with the SLN biopsy findings.
Materials and methods
Patients
Participants in the study were T1N0M0 breast cancer patients with a tumor size of 20 mm or less and no clinical lymph node metastasis, who submitted written informed consent. A total of 557 patients (median age 56 years, range 26–84 years) completed the procedures from August 2003 through January 2008.
The criteria for inclusion were detection with lymphoscintigraphy of only a single SLN, and surgical removal of a single SLN. These criteria were set in order to maintain a one-to-one correspondence between detected nodes and SLNs removed for biopsy. Correlations between lymph node counts and patient factors were investigated in 100 consecutive cases (from August 2003 through July 2005, standard patients). The correlations were used to predict SLN metastasis in the next 100 consecutive cases (from August 2005 through January 2008, predicted patients).
Lymphoscintigraphy and SLN biopsy
In each case, lymphoscintigraphy was conducted on the day prior to surgical resection of breast cancer using the following procedure. A total volume of 45 MBq/0.4 mL of 99mTc-tin colloid was injected at four intradermal sites around the tumor. At 3 h after injection, the patient was placed in a supine position with the upper extremity near the affected area elevated, the injection sites were masked with a lead plate, and lymphoscintigraphy was performed with an anterior oblique view of 30°. The device used was a gamma camera equipped with a low-energy high-resolution collimator (RC2500 IV, Hitachi Medical Corp., Tokyo, Japan). Static acquisition with a 256 × 256 matrix was conducted for 5 min.
During the surgical resection, the SLN was identified with a gamma probe (neo 2000, Neoprobe, Dublin, Ohio, USA) before dissection. The biopsy consisted of two pathological diagnoses, using imprint cytology and rapid histology. If metastasis was detected in one or both of the diagnoses, the result was considered positive; if no metastasis was observed, it was considered negative.
Correlation of lymph node counts and patient factors
To quantify the degree of SLN detection with lymphoscintigraphy, a region of interest was set with a threshold value of 50%. The lymph node count was defined as the mean count within the region of interest.
The patient sets were each divided into two groups at the median values of age, BMI, and tumor size. To determine significance with respect to the presence or absence of SLN metastasis, the lymph node counts were divided into groups of positive and negative cases, and compared. Lymph node counts were also compared for the positive and negative groups within each of the groups divided by patient factors.
The differences between groups were calculated using the unequal variance t test (Welch’s t test), with p values < 0.05 defined as statistically significant.
Metastasis prediction and verification of predictions
From the lymph node counts in the 100 correlated cases (the standard patients), three reference values were derived for prediction of SLN metastasis: the maximum and mean values in the positive groups, and the minimum value in the negative groups. In the 100 predicted cases from August 2005, counts equal to or higher than the reference values were predicted to be metastasis-negative, while those below the reference values were predicted to be metastasis-positive. Those predictions were verified through comparison with the SLN biopsy findings.
Results
Lymphoscintigraphy and SLN biopsy
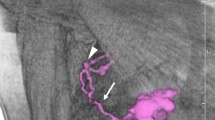
Figure 1 shows cases of SLNs with low (a) and high (b) tracer accumulations. Figure 1a is metastasis-positive with a lymph node count of 16.6. Figure 1b is metastasis-negative with a lymph node count of 135.2.
Table 1 lists the characteristics of the standard patients and the predicted patients. The median age was 53 and 56 years, respectively; the median BMI was 21.7 and 21.6, respectively; and the median tumor size was 15 mm in both sets. The patient sets thus had similar characteristics. The number of metastasis-positive SLN cases among the standard and predicted patients was 25 and 18, respectively.
Correlation of lymph node counts and patient factors
For assessment of patient factors, each patient set was divided into two at the age of 53, BMI of 21.7, and tumor size of 15 mm, and differences of lymph node counts between the pairs of groups were analyzed. Although there was a difference in age between the patient sets (p = 0.0332), no significant differences were found in BMI or tumor size. There was a clear difference between the SLN metastasis-positive and negative groups (p < 0.0001), with lower counts in the positive group (Fig. 2).
Comparisons of the lymph node counts between the metastasis-positive and negative groups divided in two according to age, BMI, or tumor size, revealed significant differences for each pair. The lymph node counts were consistently lower in the metastasis-positive groups (Table 2).
Metastasis prediction and verification of predictions
Reference values for predicting SLN metastasis were set at 10.0 (minimum value in the negative group), 19.4 (mean value in the positive group), and 53.0 (maximum value in the positive group). For the reference value of 10.0, all 11 cases with lymph node counts below 10.0 and a positive prediction were found to be metastasis-positive. For the reference value of 53.0, all 28 cases with lymph node counts equal to or higher than 53.0 and a negative prediction were found to be metastasis-negative (Table 3). Thus, the positive predictive value of the reference value 10.0 and the negative predictive value of the reference value 53.0 were both 100%. For the reference value 19.4, the sensitivity, specificity, and diagnostic accuracy were 77.8, 73.2, and 74.0%, respectively (Table 4).
Discussion
The SLN counts observed in this study were lower in the metastasis-positive group than in the metastasis-negative group. This finding suggests that lymphoscintigraphy may be used preoperatively to predict SLN metastasis.
SLN detection with lymphoscintigraphy was associated with procedural and patient factors, and correlations were made between the patient factors and the degree of detection using a fixed procedure. While a significant difference in lymph node counts was observed for age, the difference observed for presence or absence of metastasis was even more significant. Higher age, BMI, or tumor size tended to correlate with lower lymph node counts, while significant differences were clearly observed between these groups according to the presence or absence of metastasis (Table 2).
SLN detection most strongly reflected the presence or absence of metastasis, with lymph node counts being lower in the metastasis-positive group than in the negative group. To explain this finding, we may infer that radioactive tracer does not accumulate in the metastatic lesion within an SLN. Accordingly, metastatic prediction would be possible through lymph node assessment.
If readers predict metastasis using lymph node counts, they have to establish reference values. Reference values have to be assessed by lymphoscintigraphy with a fixed procedure. In addition, the reference values are assumed to be changeable by an institution even when a fixed procedure is applied.
We established the reference values reappraised from lymph node counts in the 100 cases. When the lymph node count was below 10 (minimum value in the negative group), we predicted that SLN would be metastasis-positive. And when the lymph node count was higher than 53 (maximum value in the positive group), we predicted SLN to be metastasis-negative. In fact, in the present investigation of 100 cases, positive metastasis was predicted in all 11 cases with low lymph node counts, and negative metastasis was predicted in all 28 cases with high lymph node counts, consistent with the diagnoses based on SLN biopsies.
Wang et al. [16] reported a lower SLN detection rate in patients with metastasis to the axillary lymph nodes than in patients with no metastasis, but they did not discuss the degree of SLN detection. The present study also found lower lymph node counts and less clear SLN detection in the metastasis-positive group than in the metastasis-negative group, and made a further comparative investigation of patients with and without SLN metastasis.
Nakashima et al. [17] performed dynamic lymphoscintigraphy using 99mTc-phytate, and reported that abnormal accumulation was observed near the hot spots (SLNs) in the metastasis-positive cases. In that study, patients with abnormal accumulation near the SLN were predicted to be metastasis-positive. In the present study, no abnormal accumulation was observed near SLNs because the tracer was 99mTc-tin colloid, which has a larger particle size than 99mTc-phytate. As 99mTc-tin colloid takes longer to flow into the lymph channel, it is not suitable for dynamic lymphoscintigraphy. Accordingly, for metastatic prediction with lymphoscintigraphy using 99mTc-tin colloid, lymph node counts should be assessed. Whereas Nakashima et al. based the metastatic prediction on visual assessment, this study used measurement and quantitative evaluation of lymph node counts to improve the accuracy of prediction.
Preoperative prediction of SLN metastasis in breast cancer is possible with either the dynamic lymphoscintigraphy employed by Nakashima et al., or lymphoscintigraphy as used in the current study. Thus, although preoperative lymphoscintigraphy was initially explored for the sole objective of SLN detection, in the future it will likely provide information useful for metastatic prediction.
The present study was limited to patients with only a single SLN, and there is a need to investigate cases with two or more SLNs in the future. Martin et al. [18] have already reported that when metastasis-positive and metastasis-negative lymph nodes were mixed in patients with multiple SLNs, metastasis was found in the lymph nodes with lower counts. This suggests that metastatic prediction will also be possible in cases with multiple SLNs.
Conclusion
The SLN counts detected with lymphoscintigraphy were significantly lower in metastasis-positive lymph nodes than in metastasis-negative lymph nodes. This strongly suggests that prediction of SLN metastasis in breast cancer is possible using lymphoscintigraphy.
References
Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917–24.
Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–40.
Giuliano AE, Kirgan DM, Guenther JM, Morton D. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401.
Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer: a multicenter validation study. New Engl J Med. 1998;339:941–6.
Motomura T, Inaji H, Komoike Y, Kasugai T, Nagumo S, Noguchi S, et al. Sentinel node biopsy in breast cancer patients with clinically negative lymph-nodes. Breast Cancer. 1999;6:259–62.
Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–7.
Noguchi M. Sentinel lymph node biopsy and breast cancer. Br J Surg. 2002;89:21–34.
Imoto S, Murakami K, Ikeda H, Fukukita H, Moriyama N. Mammary lymphoscintigraphy with various radiopharmaceuticals in breast cancer. Ann Nucl Med. 1999;13:325–9.
Yeung HW, Cody HS, Turlakow A, Riedel ER, Fey J, Gonen M, et al. Lymphoscintigraphy and sentinel node localization in breast cancer patients: a comparison between 1-day and 2-day protocols. J Nucl Med. 2001;42:420–3.
McMasters KM, Wong SL, Martin RAG, Chao C, Tuttle TM, Noyes RD, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multi-institutional study. Ann Surg. 2001;233:676–87.
Kim R, Osaki A, Kojima J, Toga T. Significance of lymphoscintigraphic mapping with Tc-99m human serum albumin and tin colloid in sentinel lymph node biopsy in breast cancer. Int J Oncol. 2001;19:991–6.
Pelosi E, Bello M, Giors M, Ala A, Giani R, Bussone R, et al. Sentinel lymph node detection in patients with early-stage breast cancer: comparison of periareolar and subdermal/peritumoral injection techniques. J Nucl Med. 2004;45:220–5.
Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227:645–53.
Haigh PI, Hansen NM, Giuliano AE, Edwards GK, Ye W, Glass EC. Factors affecting sentinel node localization during preoperative breast lymphoscintigraphy. J Nucl Med. 2000;41:1682–8.
Cox CE, Dupont E, Whitehead GF, Ebert MD, Nguyen K, Peltz ES, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J. 2002;8:88–91.
Wang L, Yu J, Wang Y, Zuo W, Gao Y, Fan J, et al. Preoperative lymphoscintigraphy predicts the successful identification but is not necessary in sentinel lymph nodes biopsy in breast cancer. Ann Surg Oncol. 2007;14:2215–20.
Nakashima K, Kurebayashi J, Sonoo H, Tanaka K, Ikeda M, Shiiki S, et al. Preoperative dynamic lymphoscintigraphy predicts sentinel lymph node metastasis in patients with early breast cancer. Breast Cancer. 2010;17(1):17–21. doi:10.1007/s12282-009-0123-y.
Martin RCG, Edwards MJ, Wong SL, Tuttle TM, Carlson DJ, Brown CM, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. Surgery. 2000;128:139–44.
Acknowledgments
The authors thank Prof. Teruhiko Takayama for supervising our study for many years. We also express our gratitude to Dr. Hideo Inaji, Dr. Yoshifumi Komoike, and Dr. Masato Ishitobi of the Department of Surgery for their cooperation in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noguchi, A., Onoguchi, M., Ohnishi, T. et al. Predicting sentinel lymph node metastasis in breast cancer with lymphoscintigraphy. Ann Nucl Med 25, 221–226 (2011). https://doi.org/10.1007/s12149-010-0459-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-010-0459-6