Abstract
Differential mobility spectrometer is a powerful tool used for detection, filtration and characterization of ions in gas-phase. DMS instrumentation analytical performance is a matter of importance for practical application. This paper is devoted to the improving of the planar DMS analytical characteristics. The goal is to optimize ion transmission and separation efficiency for the best possible DMS performance, balanced between sensitivity and selectivity. Analytical characteristics of the DMS instrument depend on a number of interrelated parameters. Present paper focuses on the sensor geometry and transport gas flow rate and its influence on the DMS performance. To find optimal sensor design parameters a systematic approach to the DMS performance is provided and evaluated both theoretically and experimentally. To facilitate DMS optimization special criterion quantitatively describing DMS analytical quality is proposed. DMS instrumental parameters maximizing analytical quality are determined. Theoretical analysis is validated by comparison with experimental data. Practical recommendations following from these finding are presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Differential Mobility Spectrometry (DMS) discovered in the 1980’s [1–3] can be applied to the separation and characterization of gas-phase ions from any type of ion source. Over the past decades [4, 5] DMS analyzers (also known as Field-Asymmetric Ion Mobility Spectrometry, FAIMS [6]) have been applied for the analysis of a variety of chemicals at trace levels including explosives [7], drugs, chemical warfare agents [8], toxic and industrial components, sulfur contained chemicals, and other organic [9] and inorganic [10] substances. Sensitive and rapid DMS provides a unique type of selectivity that is largely orthogonal to other gas analytical techniques such as Mass Spectrometry (MS), Gas Chromatography (GC), and Ion Mobility Spectrometry (IMS). As a result, adding DMS-based instrumentation to other technologies can enhance separation, identification and quantification, since distinct physical properties are combined [11]. Hybrid DMS instruments DMS-MS [12], GC-DMS [13], DMS-IMS [14] have been reported.
DMS is a method for the selective filtration of gas phase ions according to the difference between the ion mobility at high and low electric fields, exploiting the fact that ion mobility values depend in a chemically-specific way on the applied field strength. Under near-atmospheric pressure conditions, ions in an electric field rapidly reach a limiting velocity determined by the drag on ion motion caused by scattering from the ion-neutral interaction potential. The relationship between ion velocity and field, V(E) = K(E)E, contains a proportionality constant, K(E), known as the ion mobility coefficient, which is dependent on the field strength at high fields. This effect is characterized by the normalized field dependence of ion mobility, known as the alpha function, α(E) = (K(E)-K(0))/K(0), a characteristic ion property similar to the low field ion mobility in IMS or m/z in mass spectrometry. DMS experimental data obtained and reported during the last decade demonstrates that α(E), determined by the interaction of the ion species with neutrals, is a unique ion property and therefore can be used for ion separation and identification.
A DMS-based instrument, the Differential Mobility Spectrometer, is a tunable ion filter separating ions by their trajectories in a narrow analytical channel. This analytical channel is formed between two parallel electrodes, where positive and negative ions are introduced continuously from the ion source and drawn through by the gas flow. A periodic strong asymmetric high frequency separation field combined with a weak DC compensation field is applied transverse to the flow direction. All ion species undergo an oscillatory motion transverse to the flow due to the applied field. Because of the waveform asymmetry, ions can also acquire a net transverse velocity due to difference in field strength for different parts of the waveform. Field dependence of the ion mobility (alpha function) determines the rate at which ions move transverse to the flow. A superimposed DC compensation field is tuned to compensate for this transverse motion, allowing ion species with a particular alpha dependence to be selected. The selected ions pass through the DMS analytical channel without being neutralized, and can be detected in any detector type, including mass spectrometers. Net transverse velocity of the other ion species will not be compensated, so the ions will be neutralized on one of the electrodes inside the analytical channel. Adjusting the compensation field and the separation field magnitudes establishes conditions for filtration for all ion species with different alpha parameters.
Analytical performance of the DMS instrument depends on a number of interrelated parameters: height, width, and length of the planar analytical channel; drift gas flow rate; waveform frequency and shape, and amplitude of the separation field, as well as temperature, pressure, and composition of the drift gas. Analytical channel and electric field affect DMS separation, whereas the gas-phase environment can modify the alpha parameter itself through the reversible clustering [15].
DMS optimizations in terms of the separation field and alpha modification have been the subject of previous publications [16, 17]. The present paper focuses on the DMS analytical channel, and its influence on the DMS performance. The challenge is to choose appropriate analytical channel parameters optimally balanced between sensitivity and selectivity. We aim here to provide a systematic approach to the DMS performance, to evaluate it both theoretically and experimentally, and, furthermore, to find optimal instrumental parameters maximizing it. Here we consider only planar geometry of the DMS sensor.
To solve that optimization task we first propose optimization criterion quantitatively describing DMS analytical quality. Then based on the DMS operation model we find instrumental parameters to maximize the analytical quality. And finally we present experimental data verifying our theoretical analysis.
DMS analytical quality
In this section, we formalize DMS optimization task by proposing a quantitative criterion of the DMS analytical quality. As any spectrometer DMS provide information in a form of a spectrum. DMS spectrum peak is characterized by the peak intensity, peak position and peak width. Peak position may vary within a certain range called the analytical space. DMS resolution is defined as the range of peak positions divided by the peak width. Responsivity measures the input–output gain of a detector system. In the case of DMS, responsivity is the output ion current divided by the input molecular flow.
Both resolution and responsivity (commonly called selectivity and sensitivity) characterize DMS analytical quality, but neither one nor another can serve as an optimization criterion. One characteristic can be improved at the expense of another. For instance, DMS instrument of high responsivity but low resolution had been reported in [18], while the converse, resolution can be significantly increased by sacrificing responsivity [19].
To solve optimization task we suggest here general criterion of the analytical quality equal to the product of resolution and responsivity. This criterion reflects a balanced trade-off between selectivity and sensitivity of the DMS instrument and may serve as an optimization objective.
Specifically for the optimization of the DMS analytical channel analytical quality criterion should be narrowed. Notice that peak position (for the same separation field and alpha) and input ion flux (for the same ion source and flow) are independent of the analytical channel parameters. Analytical channel affects the resolution only through the peak width and the responsivity only through the ion losses. We characterize ion losses in the DMS analytical channel by the ion transmission coefficient equal to the output ion flux divided by the input ion flux. Thus modified DMS analytical quality in application to the analytical channel optimization is equal to the ratio of the ion transmission coefficient and peak width.
At this point we can make the optimization task statement: we are seeking for the DMS instrumental parameters (namely height, width, length of the analytical channel and drift gas flow rate) maximizing modified analytical quality. To solve this optimization task we employed theoretical approach validated by experimental data.
Theoretical optimization
The optimization defined in the previous section can be achieved within the framework of the DMS operation model [20–22] Below we use following terms and definitions: K and D are the ion mobility and diffusion; S, F, and f are the separation field amplitude, frequency, and waveform respectively; H and W are the DMS peak height and width; d, l, and c are the height, length and width of the DMS analytical channel; Q is the drift gas flow rate; e is the base of the natural logarithm. Separation field and alpha dependence are considered fixed while geometric parameters and gas flow rate are varied.
Two parameters are important for optimization of the analytical channel: effective gap g and residence time t res . The effective gap between electrodes is smaller then the physical gap because the ions, approaching electrodes to a distance equal to the amplitude of their fast oscillations, neutralize.
Average time ions spend in the analytical channel is called residence time.
For the Ion transmission coefficient G and DMS peak width W we provide here only the final expressions necessary for the solution without details which can be found in the DMS-model papers. Ion transmission coefficient, G, is a ratio of the ion flux at input and output of the analytical channel. G = 0 corresponds to total ions loss; G = 1 corresponds to lossless passage through the analytical channel. For planar DMS ion transmission coefficient is
Equating the ion displacement during the residence time to the effective gap yields DMS peak width
Combining the last two equations yields modified DMS analytical quality A = G/W as a function of the residence time.
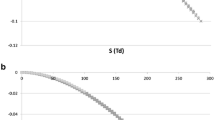
In Fig. 1 DMS analytical quality is plotted against the residence time for a) different distances between the separation electrodes (ion mobility 2 cm2/Vs) and b) for different mobilities (distance between the separation electrodes 0.75 mm). Separation field amplitude 45 Td; frequency 1.25 MHz; fly back waveform were used for the calculations. This dependence has clear extreme at residence time topt
where DMS analytical quality reaches the maximum Amax.
For the same ion, analytical quality appears to be proportional to the effective gap. That is consistent with modern trend of increasing distance between the separation electrodes up to 1 mm [23]. Given effective gap determines optimal residence time. Both effective gap and residence time determines optimal dimensions of the DMS analytical channel and gas flow rate according to Eq. 2.
Modified DMS analytical quality defined as ratio of the ion transmission coefficient and peak width in dependence of the residence time for a) different distances between the separation electrodes (ion mobility 2 cm2/Vs) and b) for different mobilities (distance between the separation electrodes 0.75 mm). Separation field amplitude 45 Td; frequency 1.25 MHz; fly back waveform were used for the calculations
The ion transmission coefficient at maximum analytical quality is equal 1/e ≈ 0.368 (by substituting Eq. 6 into Eq. 3). Substituting Eq. 6 into Eq. 4 yields DMS peak width at maximum analytical quality
Taking into account Generalized Einstein Relation Eqs. 7 and 8 can be simplified as
where q i is ion charge; T eff is average effective ion temperature.
Experimental
Thus theory predicts existing of the maximum DMS analytical quality and its dependence on the residence time, effective gap and ion properties. To validate our theoretical findings we conducted laboratory experiments involving three DMS sensors and two ion species. The goal was to measure actual DMS analytical quality in a wide range of the experimental conditions and to compare experimental data and theory. We measured height and width of single DMS peak in dependence of the gas flow rate for different distances between separation electrodes. Raw data (gas flow rate, distance between separation electrodes, peak height and width) then had been processed to compare with the theoretical values (analytical quality, residence time, and effective gap). Gas flow rate and distance between separation electrodes govern the residence time and effective gap. DMS peak height and width can be used to calculate DMS analytical quality. To test effect of the ion diffusion the same measurements were repeated for the ions of different mobility.
The ion species under investigation were: positive reactant ions identified as (H2O) n H+, and positive Methyl Salicylate ions MH+ ionized by 63Ni beta-source in clean air of temperature 60 C. For the ion identification DMS analyzer was joined to the Waters Micromass ZQ single-quadrupole system in a way described previously [24]. Air was purified by a Drierite Gas Purifier (W.A. Hammond Drierite Co., Ltd., Xenia, OH) and used as a transport gas to move ions through the DMS analytical channel. Standard Sionex DMS (model SVAC-G) was modified to drive custom sensors distinguished only by the distance between the separation electrodes: 0.5, 0.75, and 1 mm. Other dimensions of the analytical channels were the same: length 10 mm and width 4 mm. Separation voltage amplitude was 500 V, 750 V, and 1000 V for 0.5 mm, 0.75 mm, and 1.0 mm separation gaps respectively. Drift gas flow rate was scanned in a range from 60 to 500 cm3/min. Diffusion limit of the DMS peak width determines the lower flow rate value. Ionization source saturation determines upper flow rate value. Origin 7.5 (OriginLab Corporation, Northampton, MA) was used to extract peak heights and widths from the DMS spectra. Least-squares-optimized values for the peak intensities and peak widths are plotted in Fig. 2 against the gas flow rate.
To obtain the ion transmission coefficient DMS peak height should be normalized to the input ion flux. Positive reactant ions (H2O) n H+ appears to be the most suitable object for the experimental measurement of the ion transmission coefficient. It was found that experimental function Qln(H/Q) is linear in respect to the gas flow rate. It means that under present experimental conditions input reactant ion flux is proportional to the gas flow rate. Ion transmission coefficient and Input ion flux determine the line slopes and axe intersections respectively. This fact allows accurate calculation of the ion transmission coefficient (see Fig. 3). For the correct comparison of the ion transmission coefficients for different sensors the same ion source was used for all three DMS sensors.
DMS peak width (see Fig. 2b) depends linearly on the gas flow rate in agreement with Eq. 4. Poorer agreement was observed between the experimental peak width value and predictions of Eq. 4: The DMS peak was observed to be wider than expected. This means that simple DMS peak width expression should be refined for better quantitative agreement with experimental data, although it is out of scope of this article. However all conclusions related to the DMS sensor optimization remain valid because DMS peak width depends linearly on the gas flow rate.
Combining experimentally obtained ion transmission coefficient and peak width yields analytical quality dependence on the residence time (see Fig. 4). Experimentally measured analytical quality appears to be in a satisfactory agreement with theoretical predictions. Optimal residence time for positive reactant ions was 3, 7, and 12 ms for 0.5, 0.75, and 1 mm separation gaps respectively.
Optimal residence time is expected to depend on the ion mobility (see Fig. 1b). To verify this effect, positive reactant ions and positive Methyl Salicylate ions had been compared. A stable flow of vapor samples of methyl salicylate was provided by model 190 Dynacalibrator calibration gas generators (VICI Metronics Inc., Poulsbo, WA). A flow of dry nitrogen at 5 L/min was passed through the permeation tube chamber containing permeation tubes supplied by Kin-Tek Laboratories, Inc. (LaMarque, TX). The generator chamber was at 80 °C. A small portion (3–35 mL/min) of total gas flow from the gas generator was introduced into the DMS analyzer. The concentration of sample vapors after dilution in transport gas was about 0.5 ppbv. Due to charge transfer reactions with reactant ions, analyte molecules form ions MH+(H2O)n. Experiment confirms small shift of the optimal residence time attributed to the difference in mobilities of the reactant and methyl salicylate ions. For methyl salicylate ions optimal residence time appears to be 4, 10, and 16 ms for 0.5, 0.75, and 1 mm separation gaps respectively. That is about 30 % higher than for the reactant ions (see Fig. 4).
Conclusion
An analytical quality criterion reflecting a balanced trade-off between DMS selectivity and sensitivity has been proposed. The proposed criterion was utilized for the optimization of DMS performance. Analytical quality was found to have maxima at certain DMS parameters. For the same ion, analytical quality is proportional to the effective filter gap. So, in general, increasing of the distance between the separation electrodes results in the increasing of the DMS analytical quality. It should be noted that some applications may require elevated sensitivity or selectivity. Therefore the best DMS parameters for that application may differ from the ones proposed here. But in any case, the relations between the DMS parameters given here can be recommended as a good starting point. Small variation of the gas flow rate and/or DMS analytical channel dimensions around the optimal values would likely satisfy any reasonable requirements to the DMS performance.
References
Gorshkov MP (1982) S.U. Patent No. 966,583 G01N27/62
Buryakov IA, Krylov EV, Soldatov VP (1989) S.U. Patent No. 1485808, G01N27/62
Buryakov IA, Krylov EV, Soldatov VP (1988) S.U. Patent No.1 412447 G 01N 27/62
Buryakov IA, Krylov EV et al (1993) Int J Mass Spec Ion Process 128:143–148
Shvartsburg AA (2009) Differential ion mobility spectrometry: nonlinear ion transport and fundamentals of FAIMS. CRC, Boca Raton
Purves RW, Guevremont R et al (1998) Rev Sci Instrum 69:4094
Eiceman GA, Krylov E et al (2004) Anal Chem 76:4937
Krylova N, Krylov E et al (2003) J Phys Chem A 107:3648
Eiceman GA, Miller RA et al (2001) J Chromatogr A 917:205
Eiceman GA, Krylov EV et al (2004) The Analyst 129:297
Anderson AG, Markoski KA et al (2008) DMS-IMS2, GC-DMS, DMS-MS: DMS hybrid devices combining orthogonal principles of separation for challenging applications. Proc. SPIE. doi:10.1117/12.782429
Verenchikov AN, Krylov EV et al (1991) In: Malakhov VV (ed) Chemical analysis of environment. Nauka, Novosibirsk
Miller RA, Eiceman GA et al (2001) Int J Ion Mobil Spectrom 4(2):58–61
Nazarov EG, Anderson AG et al (2007) Int J Ion Mobil Spectrom 10
Krylov EV, Nazarov EG (2009) Electric field dependence of the ion mobility. Int J Mass Spectrom 285:149–156
Krylov EV, Coy SL et al (2010) Selection and generation of waveforms for differential mobility spectrometry. Rev Sci Instrum doi:10.1063/1.3284507
Schneider BB, Covey TR et al (2010) Control of chemical effects in the separation process of a differential mobility mass spectrometer system. Eur J Mass Spectrom 16:57–71. doi:10.1255/ejms.1025
Shvartsburg AA, Smith RD et al (2009) Ultrafast differential ion mobility spectrometry at extreme electric fields in multichannel microchips. Anal Chem 81:6489–6495
Shvartsburg AA, Smith RD (2011) Ultrahigh-resolution differential ion mobility spectrometry using extended separation times. Anal Chem 83:23–29
Krylov EV (2003) Comparison of the planar and coaxial field asymmetrical waveform ion mobility spectrometer (FAIMS). Int J Mass Spectrom 225(1):39–51
Krylov EV, Nazarov EG, Miller RA (2007) Differential mobility spectrometer: model of operation. Int J Mass Spectrom 226:76–85
Buryakov IA, Krylov EV, Soldatov VP (1991) In: Malakhov VV (Ed) Chemical analysis of environment. Nauka Novosibirsk
Schneider BB, Covey TR et al (2010) Planar differential mobility spectrometer as a pre-filter for atmospheric pressure ionization mass spectrometry. Int J Mass Spectrom 298(1):45–54. doi:10.1016/j.ijms.2010.01.006
Coy SL, Krylov EV et al (2010) Detection of radiation-exposure biomarkers by differential mobility prefiltered mass spectrometry (DMS-MS). Int J Mass Spectrom 291:108–117. doi:10.1016/j.ijms.2010.01.013
Acknowledgments
We thank Dr. Stephen Coy for his valuable comments and assistance in the text preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krylov, E. Differential mobility spectrometer: optimization of the analytical characteristics. Int. J. Ion Mobil. Spec. 15, 85–90 (2012). https://doi.org/10.1007/s12127-012-0099-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12127-012-0099-8