Abstract
Thyroglossal duct cysts (TGDCs) are present in ~7% of adults and develop from the midline migratory tract between the foramen cecum and anatomic location of the thyroid. Thyroid tissue can be identified in 2/3 of TGDCs, and up to 1% develop associated malignancy, 90% of which are papillary thyroid carcinoma. Cases of follicular and anaplastic carcinoma have been documented, but there are no reports of medullary thyroid carcinoma arising in a TGDC. This is presumably due to the distinct embryologic origin of parafollicular C-cells, from which medullary carcinoma arises. The goal of this study is to determine whether parafollicular C-cells are present in TGDCs. H&E sections from 41 TGDC cases were examined for thyroid tissue, thyroglossal duct remnants, ultimobranchial remnants, and parafollicular C-cells. Immunohistochemistry was performed for TTF-1 and calcitonin. Eighty three percent (34/41) of cases contained thyroid tissue on H&E and by TTF-1. No cases (0/41) had ultimobranchial remnants or parafollicular C-cells on H&E or with calcitonin. One case of papillary carcinoma in a TGDC was identified. These cases illustrate that although TGDCs often contain thyroid tissue, parafollicular C-cells are absent. Therefore, unlike other thyroid neoplasms, there is no evidence to support the possibility of medullary carcinoma arising in a TGDC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroglossal duct cysts (TGDCs) represent roughly 70% of congenital neck masses and are present in approximately 7% of adults. They develop from persistence of the midline migratory tract between the foramen cecum and anatomic location of the thyroid gland [1]. Thyroid tissue can be identified in roughly 2/3 of TGDCs upon thorough examination [2, 3]. Additionally, up to 1% of TGDCs develop an associated malignancy, 90% of which are papillary thyroid carcinoma [4]. Tall cell and follicular variants of papillary thyroid carcinoma, as well as cases of follicular, anaplastic, squamous, and adenosquamous carcinomas, have all been documented [5,6,7,8,9,10]. However, there are no case reports of medullary carcinoma arising in a TGDC, presumably due to the distinct embryologic origin of parafollicular C-cells from the ultimobranchial body [2].
Medullary thyroid carcinomas are calcitonin-producing tumors that arise from parafollicular C-cells of the thyroid gland, representing about 10% of all thyroid neoplasms [11]. In normal thyroid tissue, parafollicular C-cells aggregate in the upper and middle portions of the lateral lobes, but are not present in the isthmus or other midline structures [12]. The goal of this study is to systematically examine TGDC specimens in order to document the absence or presence of the source of medullary thyroid carcinoma, parafollicular C-cells.
Methods
After obtaining approval from the institutional review board, a retrospective analysis of TGDC cases was performed utilizing the laboratory archives. A natural language search of all surgical pathology reports at our institution from 2003 to 2015 using the keywords ‘thyroglossal cyst’ was performed. Forty one cases were identified through this search, which also provided data including patient age, gender, and final diagnoses.
Next, thorough examination of H&E slides from each case was performed to assess and document the presence of thyroid tissue, ultimobranchial body remnants, parafollicular C-cells, and any associated malignancy in each section.
Immunohistochemistry for TTF-1 and calcitonin was performed on selected blocks from the 41 cases. De-paraffinized 3.5 micron sections were stained using the Ventana Benchmark Ultra platform and Ultraview detection kit. Mouse monoclonal TTF-1 (pre-dilute clone 8G7G3/1, Ventana, Tucson, AZ) was antigen retrieved with Ultra Cell Conditioning solution 1 (CC1) for 64 min. This was followed by incubation with the primary antibody for 32 min at 37 °C, and 4 min with the Amplification kit. Rabbit polyclonal calcitonin (pre-dilute, Cell Marque, Rocklin, CA) was antigen retrieved with CC1 for 36 min, and incubated with the primary antibody for 32 min at 37 °C. Slides were then counterstained with hematoxylin, dehydrated, and cover-slipped. If present, the distribution of TTF-1 nuclear positivity and calcitonin positivity was recorded. The total number of cases exhibiting thyroid tissue, ultimobranchial body remnants, parafollicular C-cells, and malignancy was determined.
Results
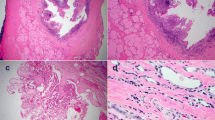
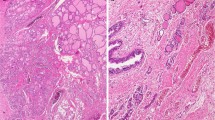
The median patient age within the case series was 33 years (range 0.1 (4 weeks)–64 years of age). The male-to-female ratio was approximately 1:1 (21 females, 20 males). Eighty three (34/41) of resected TGDCs or cyst remnants were found to contain thyroid tissue. TTF-1 staining confirmed these findings in all 34 cases, and did not reveal any additional cases with thyroid tissue remnants. Additionally, one case of papillary thyroid carcinoma arising in a TGDC was identified (Fig. 1).
In contrast, no ultimobranchial bodies or parafollicular C-cells were identified on H&E sections in all 41 cases (0/41). Furthermore, calcitonin did not highlight any ultimobranchial body remnants or parafollicular C-cells in the 41 cases examined, confirming the findings on H&E (Fig. 2).
Discussion
The lack of case reports of medullary thyroid carcinoma arising in TGDCs is likely due to absence of parafollicular C-cells in these structures, which we have systematically confirmed in our 41 patient case series using routine staining and calcitonin immunohistochemistry. Our study did identify one case of papillary thyroid carcinoma in 41 TGDCs (1/41, 2.4%), an incidence comparable to that described by Thompson et al., who reported 22 papillary thyroid carcinomas in a large series of 685 TGDCs (22/685, 3.2%) [9].
The absence of parafollicular C-cells in TGDCs can be traced back to the embryologic origin of the thyroid gland and neural crest cell migration. Thyroid follicular cells are derived from the thyroid diverticulum, which is a midline structure located near the foramen cecum at the base of the tongue. In contrast, parafollicular C-cells reside in the ultimobranchial body during development, which is derived from the fourth pharyngeal pouch. As the budding thyroid primordium begins to descend and expand laterally, the growing thyroid lobes fuse with the ultimobranchial body [12,13,14]. This process results in distribution of parafollicular C-cells in the upper and middle portions of the lateral thyroid lobes, but does not introduce C-cells into the isthmus and other midline structures [14].
The majority of parafollicular C-cells are thought to be of neural crest origin, and must migrate to the ultimobranchial body. However, recent studies have identified distinct populations of parafollicular C-cells that are of endodermal origin [15]. In either case, the embryologic origin of parafollicular C-cells is distinct from that of thyroid follicular cells. However, it is important to note that parafollicular C-cells could potentially remain along their migratory pathway near the thyroid gland and give rise to extra-thyroidal neoplasia; this could pose diagnostic difficulty in distinguishing extra-thyroidal C-cell derived tumors from metastatic laryngeal neuroendocrine neoplasms [16, 17].
During the process of primitive thyroid gland descent and expansion, it remains connected to the foregut by means of the thyroglossal duct [1]. Alterations in the process of duct involution and/or thyroid migration can result in the formation of TGDCs and other variant structures including lingual thyroid glands, pyramidal thyroid lobes, and ectopic thyroid glandular tissue [18].
In general, TGDCs are midline structures that can arise at any level from the tongue to just below the thyroid cartilage, and may display histologic features distinct from the thyroid gland proper. Histologically, the TGDC is a thin-walled, unilocular cyst lined by squamous or columnar epithelium. Accessory glandular tissue, such as thyroid or salivary gland remnants, may be present in the cyst wall, particularly when at the level of the hyoid bone [1, 5]. Prior studies have shown wide variability in the percent of TGDCs that contain remnant thyroid follicles, ranging from 45 [3] to 66% of cases [2]. Our study found that thyroid tissue was present in 83% of cases by H&E, and this finding was confirmed by immunohistochemical studies for TTF-1. Limited histologic sampling could account for differences between studies, as could variation based upon the anatomic location of the TGDC in each particular case. In this regard, it is important to note that physical exam findings and pre-operative imaging studies were not reviewed in this analysis. Therefore, it is unknown whether the increased prevalence of residual thyroid tissue in our cases is reflective of cyst anatomic location.
In contrast to TGDCs, pyramidal lobes are exclusively formed from distal thyroglossal duct remnants, and are present in about 50% of the general population, with an increased prevalence in males [19, 20]. Pyramidal lobe histology is overall comparable to other portions of the thyroid gland, although follicle size may vary by age [21]. Given that pyramidal lobes are midline structures like TGDCs, our study suggests it would be unlikely to find parafollicular C-cells in these lobes.
The retrospective nature of our analysis may have prevented review of other possible cases not captured by our natural language search. Natural language searches are limited in their ability to identify all cases, as language preferences used in final diagnoses may evolve over time and differ between pathologists. Furthermore, while the available embryologic and developmental thyroid literature supports our findings, the sample size in this study is relatively small.
In conclusion, this retrospective analysis illustrates that although TGDCs frequently contain remnant thyroid tissue, ultimobranchial bodies and parafollicular C-cells are absent. Therefore, unlike other thyroid neoplasms such as papillary thyroid carcinoma, there is no evidence to support the possibility of medullary thyroid carcinoma arising in the setting of a TGDC. These findings are consistent with the literature to date.
References
Chou J, Walters A, Hage R, et al. Thyroglossal duct cysts: anatomy, embryology and treatment. Surg Radiol Anat. 2013;35(10):875–81. doi:10.1007/s00276-013-1115-3.
Mazzaferri EL. Thyroid cancer in thyroglossal duct remnants: a diagnostic and therapeutic dilemma. Thyroid. 2004;14(5):335–6. doi:10.1089/105072504774193140.
Livolsi VA, Perzin KH, Savetsky L. Carcinoma arising in median ectopic thyroid (including thyroglossal duct tissue). Cancer. 1974;34(4):1303–15. doi:10.1002/1097-0142(197410)34:4<1303::AID-CNCR2820340442>3.0.CO;2-S.
Motmed M, McGlashan JA. Thyroglossal duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):106–9. doi:10.1097/00020840-200404000-00009.
Wei S, LiVolsi VA, Baloch ZW. Pathology of thyroglossal duct: an institutional experience. Endocr Pathol. 2015;26(1):75–9. doi:10.1007/s12022-015-9354-y.
Chang Y-S, Su H-H, Ho S-P. Adenosquamous carcinoma arising from a thyroglossal duct cyst: a case report. Oncol Lett. 2016;11(4):2668–72. doi:10.3892/ol.2016.4262.
Deshpande A, Bobhate SK. Squamous cell carcinoma in thyroglossal duct cyst. J Laryngol Otol. 1995;109(10):1001–4. http://www.ncbi.nlm.nih.gov/pubmed/7499934.
Mobini J, Krouse TB, Klinghoffer JF. Squamous cell carcinoma arising in a thyroglossal duct cyst. Am Surg. 1974;40(5):290–4. http://www.ncbi.nlm.nih.gov/pubmed/4821352.
Thompson LDR, Herrera HB, Lau SK. A clinicopathologic series of 685 thyroglossal duct remnant cysts. Head Neck Pathol. 2016;10(4):465–74. doi:10.1007/s12105-016-0724-7.
Kwan WB, Liu FF, Banerjee D, Rotstein LE, Tsang RW. Concurrent papillary and squamous carcinoma in a thyroglossal duct cyst: a case report. Can J Surg. 1996;39(4):328–32. http://www.ncbi.nlm.nih.gov/pubmed/8697325.
Utiger RD. Medullary thyroid carcinoma, genes, and the prevention of cancer. N Engl J Med. 1994;331(13):870–1. doi:10.1056/NEJM199409293311309.
Schmid KW. Histopathology of C cells and medullary thyroid carcinoma. Recent Results Cancer Res. 2015;204:41–60. doi:10.1007/978-3-319-22542-5_2.
Donoghue PCJ, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30(6):530–41. doi:10.1002/bies.20767.
Fagman H, Nilsson M. Morphogenesis of the thyroid gland. Mol Cell Endocrinol. 2010;323(1):35–54. doi:10.1016/j.mce.2009.12.008.
Johansson E, Andersson L, Ornros J, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142(20):3519–28. doi:10.1242/dev.126581.
Hirsch MS, Faquin WC, Krane JF. Thyroid transcription factor-1, but not p53, is helpful in distinguishing moderately differentiated neuroendocrine carcinoma of the larynx from medullary carcinoma of the thyroid. Mod Pathol. 2004;17(6):631–6. doi:10.1038/modpathol.3800105.
Smets G, Warson F, Dehou MF, et al. Metastasizing neuroendocrine carcinoma of the larynx with calcitonin and somatostatin secretion and CEA production, resembling medullary thyroid carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990;416(6):539–43. doi:10.1007/BF01600306.
Sameer KSM, Mohanty S, Correa MMA, Das K. Lingual thyroglossal duct cysts—a review. Int J Pediatr Otorhinolaryngol. 2012;76(2):165–8. doi:10.1016/j.ijporl.2011.11.025.
Milojevic B, Tosevski J, Milisavljevic M, Babic D, Malikovic A. Pyramidal lobe of the human thyroid gland: an anatomical study with clinical implications. Rom J Morphol Embryol. 2013;54(2):285–9. http://www.ncbi.nlm.nih.gov/pubmed/23771071.
Prakash, Rajini T, Ramachandran A, Savalgi GB, Venkata SP, Mokhasi V. Variations in the anatomy of the thyroid gland: clinical implications of a cadaver study. Anat Sci Int. 2012;87(1):45–9. doi:10.1007/s12565-011-0115-9.
Sadat A, Nurunnabi M, Alim A, Mahbub S, Kishwara S. Morphological and histological study of the pyramidal lobe of the thyroid gland in Bangladeshi people—a postmortem study. Bangladesh J Anat. 2009;7(2):94–100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Rights and permissions
About this article
Cite this article
Stein, T., Murugan, P., Li, F. et al. Can Medullary Thyroid Carcinoma Arise in Thyroglossal Duct Cysts? A Search for Parafollicular C-cells in 41 Resected Cases. Head and Neck Pathol 12, 71–74 (2018). https://doi.org/10.1007/s12105-017-0826-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-017-0826-x