Abstract
The most recent A.F.I.P. fascicle defines primordial odontogenic cyst (POC) as a distinct, nonkeratinized, odontogenic cyst of “undetermined origin” forming in the place of a developing normal or supernumerary tooth. However, the majority of examples reported in the literature under this term represent odontogenic keratocysts (keratocystic odontogenic tumors). In addition, there are rare reported cases of cystic odontomas. An 18-year-old Caucasian male presented with a unilocular mandibular radiolucent lesion in the place of a congenitally missing molar. Histologically, it featured nonkeratinizing, thin stratified squamous epithelial lining with areas of spongiosis and foci of vacuolization of individual basal cells without significant nuclear palisading. Focally, budding of the basal cell layer was identified. A zone of increased cellularity featuring induction-type fibroblasts was present subepithelially as well as dentinoid deposits with odontogenic epithelial nests. Immunohistochemically, the epithelial lining was negative for calretinin and the induction-like zone negative for S100 protein, smooth muscle actin, and CD34. The case was externally reviewed by five oral pathologists who provided various diagnostic interpretations including primordial cyst, odontogenic cyst not otherwise specified (NOS), cyst with ameloblastic changes, and unicystic ameloblastoma. At that time, a final diagnosis of odontogenic cyst NOS was rendered with a comment that it may represent a true example of POC or a cystic odontoma. The lesion has not recurred within a 13 year follow-up period after initial excision. An unusual cystic lesion is presented that may represent a true example of POC with dentinoid formation or an archegonous cystic odontoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rarely, there are odontogenic lesions that cause difficulties in interpretation since accepted classifications do not include such paradigms. However, such lesions are of great interest, leading to fruitful discussion about their pathogenesis and debate on the appropriate term to be used for their designation. Herein, we report the clinical, histologic, and immunohistochemical properties of an unusual cystic lesion.
Case Report
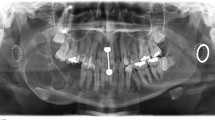
In 2001, an 18-year-old Caucasian male presented with a unilocular and expansile radiolucent lesion, measuring 1.8 cm in greatest dimension, occupying the space between the first (tooth #30) and third impacted (tooth #32) right mandibular molars. The second right mandibular molar (tooth #31) was congenitally missing and no previous history of tooth extraction in this area was reported. The cyst appeared to have caused displacement of the third molar into the mid-ramus (Fig. 1). An excisional biopsy was performed. Despite the radiographic association of the cyst with the crown of the third molar, a complete bone separation of the cyst from the impacted molar was noticed during surgery.
Macroscopically, the specimen consisted of a light tan and white semi-translucent cystic fragment measuring 2.5 cm in greatest dimension. Histopathologic examination revealed a cystic cavity lined by non-keratinizing, generally thin stratified squamous epithelium characterized by small cuboidal and polygonal cells (Fig. 2a). Elongated or spindle cells were also seen. The superficial layers exhibited spongiosis and there were foci of lymphocytic and neutrophilic exocytosis (Fig. 2b). Areas of vacuolization of individual basal cells and focal subtle palisading of the basal cell layer were appreciated (Fig. 2c) as were cord-like epithelial extensions of the basal cell layer into the supporting cystic wall (Fig. 2d). This wall exhibited throughout a subepithelial cellular, induction-type, fibroblastic zone (Fig. 2e, f). Beneath that zone, the connective tissue was less cellular. Focally, this proliferation was lobular and ameloblastic fibroma-like with small nests of odontogenic epithelium identified in the periphery (Fig. 3a, b) and were reminiscent of what is accepted as developing odontoma. In addition to the fibroblastic proliferations, there were deposits of hyalinized dentinoid-like (without obvious dentinal tubules) collagenous extracellular matrix. The dentinoid-like material was characterized by haphazard arrangement of collagen fibers, as disclosed by polarized light, and featured focal calcifications (Fig. 3c). Scattered thin, irregular cords and nests of odontogenic epithelium with frequent cytoplasmic vacuolization were present within the dentinoid-like deposits (Fig. 3c). Isolated aggregates of odontogenic epithelium with rare squamous metaplasia were identified (Fig. 3d).
a Low-power photomicrograph depicting a fibrous connective tissue wall of varying thickness lined by thin epithelium. b Spongiosis and foci of lymphocytic and neutrophilic exocytosis were seen in the superficial epithelial layers. c Areas of vacuolization of individual basal cells and focal subtle palisading of the basal cell layer were appreciated, as well as cords of odontogenic epithelium in the connective tissue, demonstrating basaloid cells with vacuolar degeneration. d Medium-power photomicrograph showing cord-like epithelial extensions of the basal cell layer into the supporting cystic wall. e, f Medium- and high-power photomicrograph revealing a subepithelial, cellular, induction-type, fibroblastic zone (H&E, original magnification a ×16, b ×280, c ×420, d ×360, e ×220, f ×480)
a, b Low- and high-power photomicrograph exhibiting a lobular and ameloblastic fibroma-like proliferation with small nests of odontogenic epithelium in the periphery of the cystic wall. c Hyalinized dentinoid-like deposits with scattered nests of odontogenic epithelium were observed. d Isolated aggregates of odontogenic epithelium with squamous metaplasia were also identified (H&E, original magnification a ×63, b ×370, c ×190, d ×480)
Subtyping of this cystic lesion presented a diagnostic challenge. The Vickers-Gorlin [1] criteria (hyperchromatism of the basal cell nuclei with palisading and polarization of the nuclei with cytoplasmic vacuolization), were inconspicuous or absent. Hence, a diagnosis of cystic ameloblastoma, although taken into consideration, was not favored. Multiple sections were evaluated and the epithelial features were consistent in all sections. Be that as it may, to further investigate the possibility of ameloblastoma, multiple sections were immunohistochemically assessed for calretinin which can be identified in ameloblastomas but not in odontogenic cysts [2–5]. Besides calretinin, and in order to erase any doubt regarding the fibroblastic origin of spindle cells in the subepithelial induction-like zone, immunohistochemical staining for S100 protein and smooth muscle actin (SMA) were performed. A CD34 immunostaining to investigate the presence of so-called activated fibroblasts was also ordered.
Materials and Methods
The specifications of antibodies used were as follows: calretinin [calbindin2 (CALB2)] (Invitrogen; rabbit polyclonal; PAD: DC8), S100 protein (Ventana; rabbit polyclonal), SMA (Abcam; mouse monoclonal; 1A4), and CD34 (Abcam; mouse monoclonal; QBEnd/10). The Ki-67 cell proliferation marker (Novocastra; mouse monoclonal; MM1) was also assessed. All immunohistochemical stains were performed on a Ventana NexES automated system (Ventana Medical Systems, Tucson, AZ) according to the manufacturers’ instructions with appropriate positive controls. To assess the positive staining pattern of calretinin, 5 ameloblastomas were used as positive controls. Two cases were classified as ‘conventional’ multicystic ameloblastomas, and three as unicystic variants; two with mural involvement and one with prominent intraluminal component. All immunostains were evaluated for their pattern of positive staining (selective, focal, diffuse) and their intensity (weak, moderate, intense). Histochemical stains Congo red and von Kossa were also performed to verify the presence of amyloid and calcium salts, respectively.
Results
The epithelial lining of the cyst was negative for calretinin (Fig. 4a, b), as were the multiple small nests of odontogenic epithelium identified at the periphery of the ameloblastic fibroma-like area (Fig. 4b inset). However, numerous dispersed positive cells were identified in the connective tissue (Fig. 4b). These cells were mast cells as confirmed by their strong immunopositivity for mast cell tryptase (Abcam; mouse monoclonal; AA1). All five ameloblastomas (5/5) showed areas of calretinin positivity, although variations in the distribution and intensity of the staining were observed (Fig. 4c–f). The clinico-pathologic characteristics of the ameloblastomas along with the calretinin immunoprofile are summarized in Table 1. The Ki-67 proliferation index of the epithelial cells lining the cyst was 0 %. Cells in the induction-like area of the cyst wall were negative for S100 protein, SMA, and CD34. Congo red histochemical stain failed to reveal the presence of amyloid, while von Kossa stain disclosed the limited presence of calcium salts.
a, b The epithelial lining of the cystic lesion was negative for calretinin, as were the small nests of odontogenic epithelium associated with the ameloblastic fibroma-like area (inset). Note, however, the multiple calretinin positive mast cells. c, d This example of multicystic ameloblastoma (Case 4, Table 1) showed areas of strong immunopositivity for calretinin. e, f An example of unicystic ameloblastoma with mural involvement (Case 5, Table 1) demonstrating strong and diffuse calretinin immunopositivity (immunoperoxidase stain, original magnification a ×420, b ×320, inset ×620, c ×120, d ×320, e ×120, f ×270)
External consulation was sought and the clinical history, histologic preparations of the specimen, and pertinent radiographs were forwarded to 5 experienced oral pathologists. The following diagnostic interpretations were provided: Odontogenic cyst not otherwise specified (NOS) (1), true POC (1), mandibular cyst with ameloblastic changes (1), and unicystic ameloblastoma (2). The senior author of this paper felt uncomfortable with the diagnosis of ameloblastoma, therefore the diagnosis of odontogenic cyst NOS was rendered with the comment that it may represent an example of true POC with areas of stromal induction and dentinoid formation. Afterwards, the senior author had the opportunity to discuss the findings with the late Dr. Robert A. Vickers who agreed with the absence of the well-known criteria. In later years, the possibility of cystic archegonous or aborted odontoma was taken into consideration as a potential diagnostic choice. Thirteen years postoperatively, there is no evidence of recurrence.
Discussion
Subclassification of this cystic lesion was undoubtedly challenging given the diverse interpretations provided by experts. The diagnosis of a somewhat controversial entity, namely, the primordial odontogenic cyst, was intriguing. In 1945, Robinson [6] defined POC as follows: “Primordial cysts are those closed epithelium lined sacs formed through degeneration of the stellate reticulum in enamel organs before any calcified structures have been laid down. They contain no calcified structures”. The latter apparently refers to enamel, dentin, and cementum. Also, Robinson [7] emphasized that the term POC was adopted in order to replace the “confusing” at that time term “follicular cyst”. The latter was applied for either tooth-bearing or even non-tooth-bearing cysts of the jaws [7].
In 1956, Robinson et al. [8] trying to establish a proper classification system for odontogenic cysts, revised the definition of POC. Segments of that publication read as follows: “The term primordial cyst was coined to describe odontogenic cysts of early origin. They are formed before any hard tooth structures are deposited…. The primordial cyst is relatively rare” [8]. In their report, no reference is made regarding the status of keratinization of the cystic epithelium. After the definition of odontogenic keratocyst (OKC) by Philipsen [9] in 1956, the terms POC and OKC were used interchangeably due to the notion that both POC and OKC originate from primordial (i.e. primitive or “original”) odontogenic epithelium [10–12].
In 1971, the W.H.O. recognized the terms OKC and POC as synonymous emphasizing primarily on their histopathologic characteristics [10, 13]. The same designation was applied to the most recent edition of W.H.O. Classification of Head and Neck Tumours, although OKC is now described under the term keratocystic odontogenic tumor (KCOT) [14]. In the latest A.F.I.P. fascicle of Tumors and Cysts of the Jaws published in 2012, POC is defined as a nonkeratinized, odontogenic cyst of undetermined origin that forms in place of a developing normal or supernumerary tooth [15]. However, the fascicle does not provide appropriate references of valid examples. On the contrary, the authors refer to publications that use the terms POC and OKC synonymously and interchangeably. Also, most likely because of an oversight, the fascicle, referring to the classic study by the late Dr. Robert Brannon [16], erronously states that 44 % of OKCs satisfy the clinical and radiographic criteria for POC (the correct number is 19 %). A careful calculation results in only 4.5 % (135/2972) of all oral cysts studied, presenting with clinico-radiographic features of POC with 44 % (60/135) being keratinizing. Examples of POC are pictured in the first, second, and third series of the A.F.I.P. fascicle [17–19]. Browne [20] and Forssell [21] have stressed the clinical descrepancies between POC and OKC, specifically the fact that only a limited number of OKCs develops in the location of a developmentally missing tooth. In a case series authored by Reff-Eberwein et al. [22] only 2 of 130 OKC examples were associated with a missing tooth. Modifications regarding the classification and nomenclature of POC in chronological order are presented collectively in Table 2.
Due to the diagnostic dilemmas that have arisen by the histopathologic characteristics of the present case, external consulation was sought. The interpretations of the consultants were divided into two groups, one supporting the diagnosis of an odontogenic cyst, the other favoring the diagnosis of unicystic ameloblastoma. One consultant merged the two by calling it mandibular cyst with ameloblastic changes. In our opinion, the so-called Vickers-Gorlin [1] criteria were not fulfilled in order to classify the lesion as ameloblastoma. However, the diagnosis of cystic lesions of the jaws similar to our example, in the absence of well-defined minimal histopathologic criteria supporting ameloblastomatous transformation, is prone to subjective interpretation. The epithelial lining of the cyst was also negative for calretinin, whilst all ameloblastomas, regardless of their type, exhibited areas of strong immunostaining. This observation is regarded by us as additional evidence to further support the diagnosis of odontogenic cyst rather than that of a unicystic ameloblastoma.
Calretinin, also known as calbindin 2 (CALB2), is a 29-kDa calcium-binding protein that has been detected in the odontogenic epithelium during odontogenesis in rat molar tooth germs [23]. Interestingly, calretinin appears to be a specific immunohistochemical marker for neoplastic ameloblastic epithelium since it has been identified in 93.5 % of multicystic and 81.5 % of unicystic ameloblastomas [2] but not in odontogenic cysts [3]. In the study by DeVilliers et al. [4] comparing the calretinin immunohistochemical profile of ameloblastomas and OKCs (KCOTs), it was concluded that all (19/19) ameloblastomas were positive in contrast to 0/17 of the OKCs (KCOTs). Similarly, Anandani et al. [5] detected immunopositivity for calretinin in 16/20 ameloblastomas but only in 1/20 OKCs (KCOTs). The single positive OKC (KCOT) case, according to the authors, demonstrated stellate reticulum like areas and palisaded basal cell layer, and, therefore, it could be in fact a unicystic ameloblastoma [5]. Contradictory results were published by Koneru et al. [24], with the latter study showing that 90 % of ameloblastomas and 80 % of KCOTs were immunopositive to calretinin. However, the intensity of calretinin immunostaining was significantly higher in the group of ameloblastomas compared to the KCOTs [24].
An interesting feature of our case was the subepithelial cellular, induction-type fibroblastic proliferation, along with a lobular ameloblastic fibroma-like area. In 2002, Usubütün et al. [25] presented an example of ameloblastic fibroma with prominent hyalinization around the ameloblastic islands as well as foci of calcification, which featured extensive central cyst formation. In contrast to the present case, no odontogenic epithelial lining was observed in the cystic area [25].
Lastly, areas of focally and miminally calcified hyalinized or dentinoid-type collagenous matrix were evident in our case. Dentinoid deposits are occasionally found in odontogenic cysts and tumors including typical ameloblastomas [26–29]. Rarely, dentinoid formation has been described in gingival odontogenic cysts [30], as well as peripheral odontogenic tumors and hamartomatous lesions [31]. The presence of this material together with the induction-like area and the dispersed odontogenic nests raises the possibility of this cyst being associated with an aborted second molar or an archegonous cystic odontoma, and as such a cystic hamartomatous process. In such a case, dental hard tissue structures had not developed at the time of cyst excision. Cystic odontomas, although rare, have been encountered in the literature [32, 33].
Recently, the term “primordial” has been used to define an unusual tumor associated with impacted, but not congenitally absent, teeth [34]. The six examples reported of the so-called primordial odontogenic tumor (POT) display solid areas of cell-rich or myxoid ectomesenchymal tissue and are entirely surrounded by columnar or cuboidal epithelium, mimicking the inner enamel epithelium of a developing tooth. No evidence of dentin or dentinoid material was seen in any of those cases. All six were associated radiographically with the crown of an unerupted tooth, primarily 3rd molars. Examples of this entity, under different terminologies, may have been published before in previous oral pathology textbooks, or have appeared in japanese journals that are not listed in the Pubmed data base [35]. We had the opportunity to review the pictures of the japanese articles and an essentially similar case to POT was presented by Morita et al. [36] under the name odontogenic fibroma. Although intriguing, the appropriateness of the term “primordial” to designate this tumor in the presence of well-developed teeth may be a topic of future discussions.
In conclusion, we report the clinicopathologic characteristics of an unusual odontogenic cystic lesion developing at the location of a congenitally missing mandibular second molar in an 18-year-old patient and address the diagnostic dilemmas which this case has evoked. Our interpretation of the epithelial lining and its immunonegativity for calretinin, did not favor the diagnosis of ameloblastoma. We have also revisited the term “primordial odontogenic cyst” in the context of this lesion. We cannot rule out the diagnosis of a cyst associated with an aborted tooth, or that of an early developing odontoma. The lesion has not recurred within a 13 year follow-up period after initial excision.
References
Vickers RA, Gorlin RJ. Ameloblastoma: delineation of early histopathologic features of neoplasia. Cancer. 1970;26:699–710.
Altini M, Coleman H, Doglioni C, Favia G, Maiorano E. Calretinin expression in ameloblastomas. Histopathology. 2000;37:27–32.
Coleman H, Altini M, Ali H, Doglioni C, Favia G, Maiorano E. Use of calretinin in the differential diagnosis of unicystic ameloblastomas. Histopathology. 2001;38:312–7.
DeVilliers P, Liu H, Suggs C, Simmons D, Daly B, Zhang S, Raubenheimer E, Larsson A, Wright T. Calretinin expression in the differential diagnosis of human ameloblastoma and keratocystic odontogenic tumor. Am J Surg Pathol. 2008;32:256–60.
Anandani C, Metgud R, Singh K. Calretinin as a diagnostic adjunct for ameloblastoma. Pathol Res Int. 2014;2014:308240.
Robinson HBG. Classification of cysts of the jaws. Am J Orthod Oral Surg. 1945;31:370–5.
Robinson HB. Primordial cyst versus keratocyst. Oral Surg Oral Med Oral Pathol. 1975;40:362–4.
Robinson HBG, Koch WE Jr, Kolas S. Radiographic Interpretation of oral cysts. Dent Radiogr Photogr. 1956;29:61–8.
Philipsen HP. Om keratocystes (kolesteatomer) i kaeberne. Tandlaegebl. 1956;69:963–80.
Partridge M, Towers JF. The primordial cyst (odontogenic keratocyst): its tumour-like characteristics and behaviour. Br J Oral Maxillofac Surg. 1987;25:271–9.
Emerson TG, Whitlock RI, Jones JH. Involvement of soft tissue by odontogenic keratocysts (primordial cysts). Br J Oral Surg. 1972;9:181–5.
Shear M, Altini M. Odontogenic and non-odontogenic cysts of the jaws. J Dent Assoc S Afr. 1983;38:555–60.
Pindborg JJ, Kramer IRH. WHO histological typing of odontogenic tumors. Geneva: Jaw Cysts and Allied Lesions; 1971.
Philipsen HP. Keratocystic odontogenic tumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. WHO classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 306–7.
Robinson RA, Vincent SD. Primordial cyst (odontogenic cyst of undetermined origin). In: Silverberg SG, editor. AFIP Atlas of tumor pathology, fourth series, Fascicle 16, tumors and cysts of the jaws. Silver Spring, Maryland: ARP Press; 2012.
Brannon RB. The odontogenic keratocyst. A clinicopathologic study of 312 cases. Part I. Clinical features. Oral Surg Oral Med Oral Pathol. 1976;42:54–72.
Bernier JL. Simple follicular (primordial) cyst. In: AFIP Atlas of tumor pathology, section IV, Fascicle 10a, tumors of the odontogenic apparatus and jaws. Washington: ARP Press; 1960.
Hoffman S, Jacoway JR, Krolls SO. Primordial cyst. In: Hoffman S, editor. AFIP Atlas of tumor pathology, second series, Fascicle 24, intraosseous and parosteal tumors of the jaws. Washington: ARP Press; 1987.
Sciubba JJ, Fantasia JE, Kahn LB. Primordial cyst. In: Rosai J, editor. AFIP Atlas of tumor pathology, third series, Fascicle 29, tumors and cysts of the jaws. Washington: ARP Press; 2001.
Browne RM. The pathogenesis of the odontogenic keratocyst. In: Fourth proceedings of the international academy of oral pathology; 1969. p. 28.
Forssell K. The primordial cyst. A clinical and radiographic study. Proc Finn Dent Soc. 1980;76:129–74.
Reff-Eberwein G, Donath K, Schmitz R. Odontogenic keratocysts (OKC). Histologic and clinical follow-up studies. Dtsch Zahnarztl Z. 1985;40:514–20 (passim).
Mistry D, Altini M, Coleman HG, Ali H, Maiorano E. The spatial and temporal expression of calretinin in developing rat molars (Rattus norvegicus). Arch Oral Biol. 2001;46:973–81.
Koneru A, Hallikeri K, Nellithady GS, Krishnapillai R, Prabhu S. Immunohistochemical expression of calretinin in ameloblastoma, adenomatoid odontogenic tumor, and keratocystic odontogenic tumor: a comparative study. Appl Immunohistochem Mol Morphol. 2014;22:762–7.
Usubütün A, Atayar C, Basal N, Araz K. Cystic ameloblastic fibroma. Br J Oral Maxillofac Surg. 2002;40:512–4.
Ng KH, Siar CH. Odontogenic keratocyst with dentinoid formation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:601–6 (Review).
Slabbert H, Altini M, Crooks J, Uys P. Ameloblastoma with dentinoid induction: dentinoameloblastoma. J Oral Pathol Med. 1992;21:46–8.
Matsumoto Y, Mizoue K, Seto K. Atypical plexiform ameloblastoma with dentinoid: adenoid ameloblastoma with dentinoid. J Oral Pathol Med. 2001;30:251–4 (Review).
Evans BL, Carr RF, Phillipe LJ. Adenoid ameloblastoma with dentinoid: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:583–8.
Shafer WG, Hine MK, Levy BM, editors. A textbook of oral pathology. 2nd ed. Philadelphia: W.B. Saunders; 1963. p. 209–10.
Ide F, Obara K, Mishima K, Saito I, Horie N, Shimoyama T, Kusama K. Peripheral odontogenic tumor: a clinicopathologic study of 30 cases. General features and hamartomatous lesions. J Oral Pathol Med. 2005;34:552–7.
Goldberg H, Schofield ID, Popowich LD, Wakeham D. Cystic complex composite odontoma. Report of two cases. Oral Surg Oral Med Oral Pathol. 1981;51:16–20.
Thoma KH. Tumors of odontogenic origin. In: Oral pathology. A histological, roentgenological, and clinical study of the diseases of the teeth, jaws, and mouth. St. Louis: C.V. Mosby; 1941. p. 960–8.
Mosqueda-Taylor A, Pires FR, Aguirre-Urízar JM, et al. Primordial odontogenic tumour: clinicopathological analysis of six cases of a previously undescribed entity. Histopathology. 2014;65:606–12.
Ide F, Kikuchi K, Kusama K, Muramatsu T. Primordial odontogenic tumour: is it truly novel? Histopathology. 2015;66:603–4.
Morita S, Jogetsu K, Nosaka Y, et al. A case of odontogenic fibroma in the mandible of a three-year-old boy (Japanese with English abstract). Jpn J Oral Maxillofac Surg. 1994;40:935–7.
Acknowledgments
The authors are grateful to Drs. Carl Allen, Paul Freedman, Raymond Melrose, Mervyn Shear and Lee Slater for providing their expert opinion as consultants. Finally, we are indebted to Mr. Brian Dunnette (University of Minnesota) for his assistance with the illustrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Argyris, P.P., Wetzel, S.L., Pambuccian, S.E. et al. Primordial Odontogenic Cyst with Induction Phenomenon (Zonal Fibroblastic Hypercellularity) and Dentinoid Material Versus Archegonous Cystic Odontoma: You Choose!. Head and Neck Pathol 10, 237–244 (2016). https://doi.org/10.1007/s12105-015-0640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-015-0640-2