Abstract
There is a lack of a comprehensive immunohistochemical (IHC) analysis of canalicular adenoma (CanAd), especially when combined with a description of the unique histologic features. Given the usual small biopsies, IHC may be useful in distinguishing CanAd from other tumors in the differential diagnosis. Retrospective. The patients included 54 females and 13 males (4.2:1), aged 43–90 years, with a mean age at presentation of 69.9 years. Clinical presentation was generally a mass (n = 61) slowly increasing in size (mean 38.5 months), affecting the upper lip (n = 46), buccal mucosa (n = 17) or palate (n = 4), involving the right (n = 29), left (n = 24) or midline (n = 9), without any major salivary gland tumors. The tumors ranged in size from 0.2 to 3 cm (mean 1.2 cm). Most tumors were multilobular or bosselated (76 %), often surrounded by a capsule. Histologically, the tumors were characterized by cystic spaces, tumor cords with beading, tubule formation, and by the presence of luminal squamous balls (n = 41). The cells were cuboidal to columnar with stippled chromatin. Mitoses were inconspicuous. A myxoid stroma (n = 64), sclerosis (n = 42), luminal hemorrhage (n = 51), and luminal microliths (calcifications) (n = 33) were characteristic. Nine (13.4 %) were multifocal. CanAd showed the following characteristic immunohistochemistry findings: CK-pan and S100 protein (strong, diffuse reaction); peripheral or luminal GFAP reaction; CK5/6 and p16 luminal squamous ball reaction; SOX10 nuclear reaction; cytoplasmic p63 reaction. CanAd are unique minor salivary gland tumors showing a distinct architecture and phenotype. They predilect to older women, with the majority multilobulated and affecting the upper lip, multifocal in 13 %; no major salivary gland tumors were identified. S100 protein, CK-pan, GFAP and SOX10 are positive, with luminal squamous balls highlighted by CK5/6 or p16.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canalicular adenoma (CanAd) is an uncommon benign salivary gland neoplasm. Originally thought to be derived from terminal duct origin [1, 2], there has been controversy about origin and separation from other salivary gland neoplasms. These tumors have undergone taxonomic drift, with many terms used to describe these tumors over the years (Table 1). However, it is now agreed that CanAd are a unique salivary gland tumor, separated from other monomorphic adenomas in the last two editions of the World Health Organization. Many minor salivary gland tumors (MSGTs) are frequently sampled by small or limited biopsies, and so the differential with other tumors can be challenging, resulting in incorrect classification and inappropriate management.
The English literature contains many case reports, small series, and a few larger series, all of which focus on a particular feature. A review of the English literature (1966–2014) reveals 456 well defined cases of CanAd, although these tumors are often presented as part of a clinical series of minor salivary gland tumors in general, within all benign salivary gland tumors, as part of a differential diagnosis presentation, a single institution’s experience or as a regional incidence report of salivary gland neoplasms in general (Table 2) [3–58]. In a literature review by Pires et al. [38], there were a total of 7,746 intra-oral minor salivary gland tumors: 3,988 benign and 3,758 malignant tumors. There were 249 CanAds, representing 6.2 and 3.2 % of benign tumors and all tumors, respectively.
It is the intention of this study to provide a comprehensive analysis of CanAd incorporating the use of clinical features, histologic findings, and immunohistochemical results applied to a group of 67 patients with this tumor, combined with a comprehensive review of the literature.
Methods
Eighty-seven cases of salivary gland tumors diagnosed as “canalicular adenoma” were selected from the clinical files of the authors between 1986 and 2012. Re-examination of the cases was performed in a blinded fashion, with 20 cases reclassified as basal cell adenoma (n = 16; 13: parotid; 2: upper lip; 1: buccal), pleomorphic adenoma (n = 3; 1 each, upper lip, palate and parotid) or chondroid syringoma (n = 1, upper lip). The remaining 67 cases were evaluated further. Materials within the files were supplemented by a review of the patient demographics (gender, age, race) and symptoms at presentation (asymptomatic, mass, pain) including duration. In addition, we reviewed the medical history, surgical pathology and operative reports, specifically noting exact tumor location, lateralization and tumor size (greatest dimension in centimeters). Follow-up data, available for 27 patients, included information regarding the specific treatment, the presence or absence of recurrent or persistent disease, and the current status of the disease and patient. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization (#5968) performed under the direction of Southern California Permanente Medical Group relating to human subjects in research.
Hematoxylin and eosin-stained slides from all 67 cases were reviewed, with a range of 1–5 slides reviewed per case (mean 1.2 slides), with each slide often containing multiple sections. The following specific macroscopic and histologic observations were recorded for each tumor: surface epithelium (present or absent); surface origin or involvement; surgical margin status; tumor bosselation or lobulation (Fig. 1); tumor multifocality (Fig. 1); capsule; cystic appearance (Fig. 2); beading of the neoplastic cells (Fig. 2); tubule formation (Fig. 2); nuclear chromatin distribution (stippled, coarse, vesicular or hyperchromatic); nuclear to cytoplasmic ratio; cell shape (columnar or cuboidal); presence of intraluminal squamous ball or morule (Fig. 2); reduplicated basement membrane; sclerosis; intraluminal hemorrhage; myxoid stroma (Fig. 3); histiocytes (luminal or stroma; foamy or hemosiderin/lipofuscin laden; Fig. 3); microliths (psammoma bodies, calcifications; Fig. 1); necrosis (present or absent); mitotic figures (number of mitotic figures per 10 high power fields [magnification at 40× with a 10× objective lens using an Olympus BX41 microscope]; Fig. 3); atypical mitotic figures (present or absent, and defined by abnormal chromosome spread, tripolar or quadripolar forms, circular forms, or indescribably bizarre); and the presence of other microscopic pathologic findings.
Canalicular adenoma showing: a Characteristic beading (arrows), with parallel rows joining. b Tubular growth with club-ended cords with small lumina, set in a loose stroma. c Cyst formation with edematous stroma. d Intraluminal squamous balls or morules (arrows). Note they may be free in the lumen or attached
Immunophenotypic analysis was performed in all cases with sufficient suitable material by a standardized Envision™ method employing 4 µm-thick, formalin fixed, paraffin embedded sections using a tissue microarray constructed using 2 mm cores from each case block with available material (n = 52). Table 3 documents the commercially available immunohistochemical antibody panel used (Figs. 4, 5, 6). Epitope retrieval was performed, as required by the manufacturer guidelines. Standard positive controls were used throughout, with serum used as the negative control. The antibody reactions were catalogued by location (nuclear, cytoplasmic, membrane, luminal, ball, canalicular); tumor distribution (stromal, cellular, peripheral); fraction of positive cells (focal, diffuse); and graded: absent to weak (0–1+), moderate (2+ to 3+) and strong (4+) staining. The proliferation marker was separated into ≤5 and >5 %. In situ hybridization for high-risk HPV was performed using an automated benchmark XT system (Ventana Medical Systems, Inc., Tucson, AZ). The INFORM HPV III family 16 probe cocktail, with affinity for high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66), was applied and the reaction was developed using a Hybrid Ready Detection Kit (Ventana Medical Systems, Inc., Tucson, AZ). Positive signals included punctate or diffuse reactivity within tumor nuclei. Periodic acid Schiff (PAS) with and without diastase, mucicarmine and alcian blue (Fig. 6) were performed on standard automated stainers.
A review of publications in English (MEDLINE 1966–2014) was performed, with all cases reported with clinical, histologic, immunophenotypic and/or follow-up information on CanAd evaluated and included in the review, but excluding “Quiz” or “Case of the Month” type reports. Several studies were excluded if no specific or separable information was given about CanAd or if the illustrations were not characteristic [23, 51, 55, 59–78]. Several of the cases seemed to have been included multiple times in different series reports [9, 10, 40, 79–83].
Statistical evaluation was performed using a standard statistics software package with categorical variables analyzed using Chi square tests and Fisher’s Exact tests to compare observed and expected frequency distributions. Comparison of means between groups was made with unpaired t tests or one-way analysis of variance, depending on whether there were two groups or more than two groups, respectively. Multiple comparisons were analyzed using the Tukey method and log-rank analysis. Confidence intervals of 95 % were generated for all positive findings. The alpha level was set at p < 0.05.
Results
Clinical
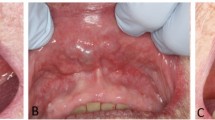
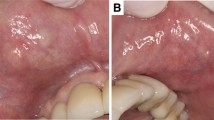
The patients included 54 women and 13 men (Table 4) who ranged in age from 43 to 90 years, with a mean age at presentation of 70 years. There was no difference in mean age at presentation between men and women, nor was there a difference in anatomic site of involvement between the genders. The majority of patients presented with a painless mass (n = 61), while 6 were discovered incidentally during dental examination. If symptomatic, symptoms were present for an average of 3 years. Multifocal tumors were identified in 9 patients, 5 of whom had another surgery at a later time to manage the tumor. The upper lip was the most common site affected (n = 46). No cases were identified in major salivary glands in this series.
Pathologic Features
Macroscopic
The tumors ranged in size from 0.2 up to 3 cm, with a mean of 1.2 cm. Tumors of the lip were on average smaller than those of the buccal mucosa or palate (p = 0.027). There was no difference in tumor size between genders. On gross examination, the tumors were partially to completely encapsulated, with a yellow-tan to brown, homogenous cut surface. The firm masses had a mucoid to gelatinous appearance, with cyst formation.
Microscopic
Microscopically, the tumors were bosselated or lobulated at the periphery (Fig. 1; Table 5), well circumscribed, occasionally showing a well developed fibrous connective tissue capsule (especially if the lesions were >1 cm). Separate tumor islands (multifocality) were noted in nine cases (Fig. 1). There was no tumor necrosis. The majority of the cases showed cyst formation (Fig. 2), frequently accompanied by intraluminal hemorrhage (n = 51) and hemosiderin/lipofucsin laden macrophages (n = 20). In a few cases the cyst was the dominant finding. Laminated calcifications, psammoma bodies, or microliths were seen in 33 cases (Fig. 1). The microliths were associated with viable cells and identified in the stroma or the lumen, often in areas with papillae. Mitoses were only found in 24 cases (Fig. 3), ranging from 1 to 4, with a mean of 0.5 per 10 high power fields, without any atypical forms. Three tumors were degenerated or had undergone infarction. One case showed tyrosine crystals (Fig. 1). The surgical margins were positive (i.e., tumor cells on ink) in 28 cases. This was not related specifically to multifocality, but rather to the lobulation or bosselations of the tumor.
The neoplastic cells were a relatively monotonous, isomorphic population of high cuboidal to columnar cells, 1–2 cell layers thick arranged in anastomosing, branching or budding cords, tubules, rows, strands, columns or canaliculi (Figs. 2, 3). Solid nests were occasionally seen. Papillary projections were common in areas of cyst formation. The rows or ribbons of cells, arranged parallel to each other, would frequently merge, creating the characteristic “beading” phenomenon (Fig. 2). Stated differently, knots of cells can be seen joining together two parallel tracks of epithelial cells that are separated by a narrow lumen or cystic space. A pseudostratification of the nuclei or vaguely palisaded appearance was seen in most cases, where there was a preferential placement of the nucleus towards the base or mid-zone of the cells (not at the lumen). This pseudostratification results from different nuclei heights and plane of section. Intraluminal squamous morules or balls were seen in 41 cases (Fig. 2). This is a unique finding not identified in other salivary gland tumors, and specifically not in basaloid neoplasms. These balls were attached or free floating, showing a “metaplastic” squamous appearance. The cells showed a moderate nuclear to cytoplasmic ratio, limited to absent pleomorphism, and lacked prominent nucleoli. The nuclei were round to oval, regular and smooth, with stippled nuclear chromatin, while focal nuclei may be vesicular or show coarse chromatin distribution (Fig. 3). The cytoplasm was moderate to scant, eosinophilic, with granules noted (highlighted with PAS-D but not mucicarmine), while rare oncocytes or mucous cells were noted (3 cases). The cells appeared as a syncytium, with indistinct cell borders. Cytoplasmic hemosiderin or lipofuscin was present at the luminal surface of isolated tumor cells in most cases (apocrine-like cells). It is important to note that a basal layer or myoepithelial layer is not present by histology (or by immunohistochemistry). A supporting loose stroma was myxoid and often associated with a sclerosing to fibrillar collagen deposition. The stroma was richly vascularized, frequently (n = 51) associated with luminal hemorrhage. No chondroid matrix or stellate/bipolar epithelial cells were present. In general, the stroma was sparse, fibrillar and edematous (Fig. 3). This stroma stained with mucicarmine, alcian blue (Fig. 6), and periodic acid-Schiff, the latter both with and without diastase. Histiocytes were present in many cases, separated into foamy histiocytes and hemosiderin/lipofuscin laden histiocytes. The foamy histiocytes were usually luminal (n = 9; Fig. 3), with two cases showing a stromal location. The lipofuscin laden macrophages were usually luminal (n = 20), although also seen in the stroma (n = 11). Only two cases had both types of histiocytes.
Immunohistochemical Results
All tumors tested reacted with S100 protein (Fig. 4), pan-cytokeratin (Fig. 5), CK7, CAM5.2, and E-Cadherin (Table 3). Additional reactive epithelial markers included CK903, EMA (Fig. 6), and CK19 (Fig. 6). GFAP was present in the majority of cases, but stained only the periphery or luminal borders in an isolated and linear fashion (Fig. 4), similar to previously reported findings [72]. p63 was negative in the nuclei of the lesional cells, but with a peculiar cytoplasmic reaction (Fig. 4). The squamous balls/morules present in the lumen were highlighted with both CK5/6, p63 (nuclear), and p16 (nuclear and cytoplasmic) (Fig. 5). p16 positive cells were non-reactive with high risk HPV. SOX10 showed a strong, patchy to diffuse nuclear reaction, while DOG1 was negative in the lesional cells. Muscle markers were negative in the tumor cells. Many other markers (such as p40, β-catenin, androgen receptor, and Her-2/neu) were also negative, performed primarily as part of the potential differential diagnostic considerations raised by evaluating small biopsy specimens.
Treatment and Follow-up
All patients were managed by surgery, with biopsy, incisional biopsy and excisional biopsy the most commonly performed procedures, rather than a wide excision or resection-type procedures. Recurrences versus persistence or multifocality were difficult to determine with certainty. Nine patients had multifocal tumors, five of whom had additional tumors removed at a later time, from a few months to decades later. No patients died of their disease and there was no malignant transformation (carcinoma ex-canalicular adenoma) (mean follow-up, 8.2 years).
Discussion
Etiology/Embryogenesis
Salivary glands contain a mixture of mucus or serous acini, which are surrounded by myoepithelial cells. The secretions are then carried by intercalated ducts to striated ducts to interlobular ducts, which empty the contents into a cavity (oral cavity, sinonasal tract, larynx, etc.). The intercalated ducts are lined by cuboidal cells, with occasional myoepithelial cells noted. The luminal cells however are considered separate from the myoepithelial cells. Striated ducts are lined by columnar cells generally lacking myoepithelial cells. Finally, the interlobular ducts have both cuboidal and columnar cells, but also nearly always lack myoepithelial cells. Over the years, the specific origin of CanAd has been postulated to arise from each of these specific duct systems [7, 10, 17, 21, 33, 84–86]. Interestingly, as most CanAd arise from the labial glands or buccal mucosa, there are very few intercalated ducts in these locations, although most intralobular ducts in minor salivary glands resemble intercalated ducts [87]. A recent publication has highlighted what has been called a striated duct adenoma, a lesion that is morphologically distinct from CA [88]. Further, Triantafyllou et al. [49] have suggested that the phenotypes in CanAd may not reflect a histogenetic origin, but may derive from the interplay between an altered secretory product, the lack of neuro-effector relationships and different microenvironments throughout the tumor.
The anoctamin-1 (ANO1, also known as DOG1) gene acts as a calcium-activated chloride channel and potentially has a role in salivary gland secretory activity. It has been recently described in normal salivary gland tissue, where DOG1 immunohistochemistry shows a strong membranous staining of normal serous acini, while intercalated ducts show an apical-luminal membranous pattern, stronger towards the distal portion as it approaches the acinus. However, striated ducts and excretory ducts are negative for DOG1 [89]. The CanAd cells were negative, without a difference seen between luminal, abluminal, peripheral or canalicular areas.
SRY-related HMG-box 10 (SOX10) protein is a transcription factor with positive expression in major salivary glands. SOX10 expression by immunohistochemistry was specific to the nuclei of normal acini and both luminal and abluminal cells of intercalated ducts, but was not identified in other sites (although positive in several tumors, such as acinic cell carcinoma, adenoid cystic carcinoma, and pleomorphic adenomas) [90]. The CanAd cells showed a strong, patchy to diffuse, nuclear reaction in nearly all of the lesional cells.
Therefore, overall, based on the histologic, immunophenotypic and ultrastructural findings, CanAd most closely approximates the phenotype of normal intercalated duct luminal cells.
Clinical Information
In many series, CanAd is the third most common minor salivary gland tumor of the oral cavity, with pleomorphic adenoma and mucoepidermoid carcinoma the most common [6, 38, 55, 91]. However, if all salivary gland tumors are taken into consideration (all sites, benign and malignant), then CanAd represent <1 % of all tumors [23, 65, 67].
The tumor is more common in women than men, although when the cases from the literature are combined with the present series, there is a female to male ratio of 1.7:1. Patients are middle age or older at presentation, with a range of 33–91 years. This tumor has not been reported in pediatric patients. While some patients are asymptomatic, most present with a painless, non-ulcerated mass, slowly growing, with an average duration of about 3 years. The clinical differential includes a mucocoele, thrombosed vessel, lipoma or other salivary gland tumor.
CanAd seems to occur exclusively in the oral labia, buccal mucosa and palate. Specifically, it occurs preferentially in the upper lip, as there are only 5 reported cases from the lower lip [25, 34, 38, 55] which are not illustrated or specifically highlighted. In general, is seems that CanAd may not develop in major salivary glands. Cases reported thus far in the major salivary glands (parotid, submandibular) are either not well illustrated or lack immunohistochemistry studies to confirm the diagnosis [25, 64, 69, 92–95]. Additional investigation is encouraged.
There is no well developed documentation of inherited or syndrome associated canalicular adenoma.
Pathology
CanAd are strikingly similar case to case. They are surrounded by a thin capsule, frequently showing lobulation or bosselations at the periphery. The capsule is better formed in larger tumors (>1 cm), and may be discontinuous. Multifocal tumors are observed infrequently (about 9 % of all cases), when combining the present series with those from the literature. Further, there can be a range from 2 to 22 separate tumors [24, 41, 42, 55]. These nodules are usually distinctly separate, several millimeters away from the main nodule of tumor. They have a similar histologic appearance to the main tumor. Some authors have referred to these as “adenomatous” growths, highlighting the lack of destructive or infiltrative growth [3, 24, 30], although they were interpreted to represent carcinoma by some [12, 45, 48]. Cyst formation of some degree is nearly always present, often containing extravasated erythrocytes, hemosiderin or lipofucsin laden macrophages, or foamy histiocytes.
Squamous balls or morules, a fairly specific feature of CA, are often present in the cystic lumen, but usually connect to the epithelium with serial sections (possibly the tips of papillae). These are thought to represent metaplasia, as these cells are uniquely CK5/6, p63, and p16 immunoreactive, findings distinctly different from the remaining tumor. However, these balls were negative with p40, suggesting the cells may not be squamous, as p40 is more specific to squamous differentiation than p63 [96]. Other tumors undergo cyst formation but do not develop these areas of metaplasia. It may be that the luminal microliths result in epithelial irritation or injury, causing a metaplastic change. However, microliths were present in 33 cases, 22 of which had luminal squamous balls; but 19 cases with luminal balls did not have microliths and 11 cases with microliths did not have luminal balls. Alternatively, there may be remodeling of the cytoskeleton in response to an increased microenvironmental pressure within the lumen [49]. Microliths are similar to sialoliths (salivary gland stones), showing lamellar calcifications [49]. It is postulated they form in phagosomes of tumor cells, as part of degradation of mucosubstances enriched by calcium. As there is no cell death, the concept of a true psammoma body (calcified necrotic cells) should not be used in this setting [49]. Luminal calcifications were associated with histiocytes in 22 cases in this series. However, lipofucsin laden macrophages were present in 7 cases that did not have microliths and 11 cases had microliths without lipofucsin laden macrophages. Further, it is known that secretory glycoproteins and calcium complexes are found in microliths in normal salivary glands [97].
The canalicular architecture, with characteristic beading is nearly diagnostic. The joining of parallel epithelial rows is quite unique to CA. The lesional cells are columnar with very limited to absent pleomorphism. Nucleoli are only focally noted. The nuclear chromatin is delicate, stippled to focally hyperchromatic. The stroma is edematous, hypocellular, fibrillar to myxoid associated with collagen deposition (sclerosis) in most cases. By histochemistry (PAS, alcian blue, mucicarmine), the mucoid to stellate reticulum-like stromal material is probably of epithelial derivation rather than connective tissue origin, although others have postulated production by tumor cells as a result of epithelial-mesenchymal transition [49]. There are PAS-positive granules in the basal area cytoplasm, confirmed by ultrastructural exam [7, 21, 33], which may give rise to the myxoid stromal material. Mitoses are rare to absent. Necrosis is vanishingly rare. Further, conspicuous by its absence is any chondroid or cartilaginous matrix material, helping to separate this tumor from a pleomorphic adenoma.
Immunohistochemical Studies
The immunohistochemistry findings reported have been mixed without any definitive series thus far. Salivary gland parenchyma tissues comprise two lineages: one epithelial luminal lineage and one myoepithelial basal lineage. Based on the findings in this series and those reported earlier, this tumor is a luminal epithelial lineage. The tumor cells show a strong reaction with epithelial markers, including AE1/AE3, CK7, CAM5.2, CK903, EMA, and CK19; reactions with CK8 and CK13 are also reported [14, 15, 21, 29, 40, 79, 98, 99]. Further, the neoplastic cells are strongly and diffusely reactive with S100 protein [14, 100], despite earlier reports of negative reactions [40, 79]. The distinctive linear reaction of GFAP at the tumor to connective tissue interface previously reported [72], was reproduced in the current series. This may be a helpful finding in separating this tumor from others in the differential diagnosis, which do not possess this unique linear or peripheral distribution. Vimentin is positive, a finding different from that reported by others [15, 29, 98], although such a strong reaction cannot be discounted by differences in technique or fixation.
Myoepithelial and muscle markers (α-smooth muscle actin, muscle specific actin, smooth muscle myosin heavy chain, calponin, desmin) are all negative [14, 80, 98]. In this series, the calponin was a weak and focal reaction, and seemed to be a “blush” rather than a genuine positive finding. Nuclear p63 is negative in the tumor cells, although positive in the areas of squamous morule formation in the lumen [101–103]. There was an aberrant cytoplasmic reaction, but the significance of this finding is unknown [104, 105].
CD117 is positive in all CanAd [106], and does not seem to help separating between CanAd, polymorphous low grade adenocarcinoma, adenoid cystic carcinoma, basal cell adenoma or pleomorphic adenoma. CD43 and bcl-2 were negative in CanAd [68, 81], a finding essentially supported by the results of this series. In this series, E-cadherin showed a strong, focal to diffuse membranous staining, different from results reported in the literature [82]. However, different manufacturers and clones were used, which may account for the differences.
SOX10 and DOG1 do not necessarily provide a unique finding, but do support the luminal intercalated duct cell phenotype [89, 90]. The differences between expression may help with the differential, as polymorphous low grade adenocarcinoma (PLGA) and pleomorphic adenoma are usually negative with DOG1, although adenoid cystic carcinoma and epithelial–myoepithelial carcinoma are often positive. SOX10 is positive in adenoid cystic carcinoma, epithelial–myoepithelial carcinoma and pleomorphic adenoma, so it may not be as useful in this differential.
Differential Diagnosis
The histologic differential diagnosis encompasses primarily salivary gland neoplasms, including basal cell adenoma, pleomorphic adenoma (PA), PLGA, adenoid cystic carcinoma (ACC), ductal adenoma, and reticulated myoepithelioma, while ameloblastoma, adenomatoid odontogenic tumor, paraganglioma, and skin basal cell carcinoma are also occasionally considered [16, 21, 25, 32, 88, 107].
Basal cell adenomas do not usually show a canalicular pattern of growth and usually show reduplicated basement membrane material. This tumor tends to occur much more commonly in the major salivary glands. In this clinical series, we identified several basal cell adenomas (subsequently excluded from further evaluation), 13 of which involved the parotid gland, with three involving the lip or buccal mucosa. Specifically, the trabecular variant is the tumor that may show morphologic similarity. Basal cell adenoma show a dual cell population with two layers, often showing cells oriented perpendicular to the basement membrane. While the tumor shows a monomorphic growth, there is strong S100 protein positivity, with p63, SMA, MSA, and SMMHC variably present. Two of the original cases from Daley et al. [10] are referred to as atypical canalicular adenoma, although not illustrated and without any reasons as to why they are atypical. However, as they were parotid gland tumors, they probably represent basal cell adenoma.
The lack of chondroid or myxochondroid matrix helps to exclude a PA. PA often shows plasmacytoid to spindled myoepithelial cells, especially in palate or buccal lesions. Further, by immunohistochemistry, the characteristic biphasic appearance is again highlighted by markers similar to basal cell adenoma. Ductal adenomas (striated duct adenoma) are usually encapsulated tumors (although multinodularity can be seen), composed of variably sized ducts without any intervening stroma and without an epithelial beading pattern or parallel tumor cords. Unlike CanAd, these tumors can be seen in the parotid gland. The cells usually have more eosinophilic (oncocytic) cytoplasm than CanAd. They are also negative with SMA, but usually positive with S100 protein, suggesting they are probably ductal adenomas [21, 88]. Reticulated myoepithelioma nearly always involve major salivary glands, may be cystic, and show polygonal to columnar cells arranged in cords and anastomosing trabeculae. Parallel cords and a beading pattern, characteristic of CanAd, are not usually found in reticulated myoepithelioma. While they overlap with S100 protein, keratin and CK7 immunoreactivity, they also show strong CK18, GFAP, calponin, p63 and SMA immunoreactivity [107]. All myoepithelial markers are negative in CanAd and are frequently positive in basal cell adenoma, myoepithelioma and PA, while the GFAP staining pattern is characteristic in CanAd.
PLGA grows in heterogeneous histologic patterns, entombing minor salivary glands and showing a characteristic perineural invasion. The neoplastic cells have more open and vesicular nuclear chromatin and show tumor cell spindling. Further, cystic change and squamous metaplasia are not features of a PLGA, and so separation from a CanAd can be achieved. ACC usually has a biphasic appearance, an infiltrative growth, significant perineural invasion, and pseudo-cystic spaces without a highly vascularized stroma. The nuclei are angulated with coarse chromatin. Mitoses are usually easily identified. This tumor also shows a strong patchy p63 reaction and a biphasic epithelial and myoepithelial phenotype.
Ameloblastoma may arise from mucosa overlying a tooth-bearing area or from the bone, although described in non-dentate sites [108]. However, most CanAd lack a stellate reticulum pattern, while also expressing a different immunohistochemistry profile.
Adenomatoid odontogenic tumors may arise peripherally from the anterior maxillary gingiva. The tumor tends to have minimal stroma, but shows cuboidal to columnar cells that may be arranged in duct-like or tubular structures, while occasionally arranged in very convoluted cords with invaginations or retiform patterns. Amorphous tumor droplets are characteristic, as is a reverse nuclear polarization away from the lumen.
Paraganglioma usually have a nested pattern, a rich vascularized stroma, tend to have significant, isolated pleomorphism, and show a characteristic neuroendocrine immunohistochemistry profile, with a delicate S100 protein sustentacular reaction. A skin basal cell carcinoma shows surface basal epidermal origin, tumor to stroma clefting or separation, a peripheral palisade, and usually easily identified mitoses. The neoplastic cell nuclei are more hyperchromatic.
Rare examples of hybrid tumors (CanAd and basal cell adenoma) may be seen. It would be important to document histologically and immunophenotypically the two tumor components. It is unclear whether they may be collision tumors (two separate tumors colliding) or true divergent differentiation tumors (such as a mixed follicular medullary carcinoma of thyroid gland). It is these authors’ practice to suggest a composite-hybrid designation, with the first name the dominant histologic finding.
One of the only studies addressing specifically frozen section diagnoses, showed CanAd are usually diagnosed correctly [95].
Treatment and Prognosis
Patients included in this report were managed at more than 25 different hospitals by dentists, surgeons, and dermatologists. Enucleation, biopsy, and excision are the treatments proposed. However, with multifocal tumors and a periphery that is bosselated to nodular, a cure is best achieved by a surgical excision that has a small cuff of normal, uninvolved parenchyma. Follow-up of the patients is recommended, at least in the short term, to manage possible multifocal disease.
Conclusion
In conclusion, canalicular adenoma is a unique minor salivary gland tumor, arising from the luminal cells of the intercalated duct. Women are affected more often than men, with a marked predilection for the 7th decade. Tumors preferentially affect the upper lip, with buccal mucosa and palate also affected, and are usually small (mean 1.2 cm), presenting as a non-painful, non-ulcerated slowly enlarging mass. About 9 % of tumors are multifocal. Histologically, tumor cell beading and intraluminal squamous balls/morules are unique, while the myxoid stroma, cystic appearance and microliths are characteristic histologic findings. Other tumors in the histologic differential diagnosis can be eliminated by a pertinent immunohistochemical panel of S100 protein, p63, CK5/6 and GFAP, the results of which would be characteristic and discriminating.
References
Batsakis JG, Luna MA, el-Naggar AK. Basaloid monomorphic adenomas. Ann Otol Rhinol Laryngol. 1991;100:687–90.
Batsakis JG. Oral monomorphic adenomas. Ann Otol Rhinol Laryngol. 1991;100:348–50.
Ahren C, Lindstrom J. Adenomatosis of accessory salivary glands of the lip. Report of two cases. ORL J Otorhinolaryngol Relat Spec. 1977;39:161–6.
Allen CM, Damm D, Neville B, et al. Necrosis in benign salivary gland neoplasms. Not necessarily a sign of malignant transformation. Oral Surg Oral Med Oral Pathol 1994;78:455–61.
Azevedo LR, Dos Santos JN, De Lima AA, et al. Canalicular adenoma presenting as an asymptomatic swelling of the upper lip: a case report. J Contemp Dent Pract. 2008;9:91–7.
Buchner A, Merrell PW, Carpenter WM. Relative frequency of intra-oral minor salivary gland tumors: a study of 380 cases from northern California and comparison to reports from other parts of the world. J Oral Pathol Med. 2007;36:207–14.
Chen SY, Miller AS. Canalicular adenoma of the upper lip: an electron microscopic study. Cancer. 1980;46:552–6.
McCoy-Collins RC, Calhoun NR, Redman RS, et al. Monomorphic adenoma of the buccal mucosa. J Oral Maxillofac Surg. 1985;43:644–8.
Daley TD. The canalicular adenoma: considerations on differential diagnosis and treatment. J Oral Maxillofac Surg. 1984;42:728–30.
Daley TD, Gardner DG, Smout MS. Canalicular adenoma: not a basal cell adenoma. Oral Surg Oral Med Oral Pathol. 1984;57:181–8.
Dayisoylu EH, Pampu AA, Mungan S, et al. Intra-mandibular canalicular adenoma: report of a rare case. J Pak Med Assoc. 2012;62:1239–41.
De la Pava S, Karjoo R, Mukhtar F, et al. Multifocal carcinoma of accessory salivary gland. A case report. Cancer. 1966;19:1308–10.
Fantasia JE, Neville BW. Basal cell adenomas of the minor salivary glands. A clinicopathologic study of seventeen new cases and a review of the literature. Oral Surg Oral Med Oral Pathol. 1980;50:433–40.
Ferreiro JA. Immunohistochemical analysis of salivary gland canalicular adenoma. Oral Surg Oral Med Oral Pathol. 1994;78:761–5.
Furuse C, Tucci R, hado de Sousa SO, et al. Comparative immunoprofile of polymorphous low-grade adenocarcinoma and canalicular adenoma. Ann Diagn Pathol. 2003;7:278–80.
Grimm EE, Rulyak SJ, Sekijima JH, et al. Canalicular adenoma arising in the esophagus. Arch Pathol Lab Med. 2007;131:1595–7.
Guccion JG, Redman RS. Canalicular adenoma of the buccal mucosa. An ultrastructural and histochemical study. Oral Surg Oral Med Oral Pathol. 1986;61:173–8.
Guedes Queiroz LM, Dantas da Silveira EJ, Silva Arruda MdL, et al. A rare salivary gland neoplasm: multiple canalicular adenoma: a case report. Auris Nasus Larynx 2004;31:189–93.
Harmse JL, Saleh HA, Odutoye T, et al. Recurrent canalicular adenoma of the minor salivary glands in the upper lip. J Laryngol Otol. 1997;111:985–7.
Havel G, Dahlenfors R, Bockman P, et al. Cytogenetical observations in a canalicular adenoma of the minor salivary glands. Hereditas. 1996;124:105–6.
Huebner TA, Almubarak H, Drachenberg CB, et al. Canalicular adenoma-search for the cell of origin: ultrastructural and immunohistochemical analysis of 7 cases and review of the literature. Ultrastruct Pathol. 2014;38:74–82.
Jansisyanont P, Blanchaert RH Jr., Ord RA. Intraoral minor salivary gland neoplasm: a single institution experience of 80 cases. Int J Oral Maxillofac Surg. 2002;31:257–61.
Jones AV, Craig GT, Speight PM, et al. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44:407–17.
Khullar SM, Best PV. Adenomatosis of minor salivary glands. Report of a case. Oral Surg Oral Med Oral Pathol. 1992;74:783–7.
Kratochvil FJ. Canalicular adenoma and basal cell adenoma. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands. Philadelphia: W.B. Saunders Company; 1990. p. 202–24.
Ledesma-Montes C, Garces-Ortiz M. Salivary gland tumours in a Mexican sample. A retrospective study. Med Oral. 2002;7:324–30.
Levine J, Krutchkoff DJ, Eisenberg E. Monomorphic adenoma of minor salivary glands: a reappraisal and report of nine new cases. J Oral Surg. 1981;39:101–7.
Maamouri F, Bellil K, Bellil S, et al. Canalicular adenoma of buccal mucosa. Pathologica. 2007;99:69–70.
Machado de Sousa SO, Soares de Araujo N, Correa L, et al. Immunohistochemical aspects of basal cell adenoma and canalicular adenoma of salivary glands. Oral Oncol. 2001;37:365–8.
Mair IW, Stalsberg H. Basal cell adenomatosis of minor salivary glands of the upper lip. Arch Otorhinolaryngol. 1988;245:191–5.
Mansueto G, Falleti J, De CR, et al. Synchronous bilateral multifocal canalicular adenoma: a case report of an unusual finding. Clin Exp Dermatol. 2009;34:e587–9.
Matsuzaka K, Murakami S, Shimono M, et al. Canalicular adenoma arising in the upper lip: review of the pathological findings. Bull Tokyo Dent Coll. 2004;45:229–33.
McMillan MD, Smith CJ, Smillie AC. Canalicular adenoma: report of five cases with ultrastructural observations. J Oral Pathol Med. 1993;22:368–73.
Nelson JF, Jacoway JR. Monomorphic adenoma (canalicular type). Report of 29 cases. Cancer. 1973;31:1511–3.
Nelson ZL, Newman L, Loukota RA, et al. Bilateral multifocal canalicular adenomas of buccal minor salivary glands: a case report. Br J Oral Maxillofac Surg. 1995;33:299–301.
Oliveira-Santos C, Freitas-Faria P, Damante JH, et al. Asymptomatic nodules of the upper lip: report of a canalicular adenoma with immunoprofile presentation. Gerodontology. 2012;29:e1121–4.
Pereira MC, Pereira AA, Hanemann JA. Immunohistochemical profile of canalicular adenoma of the upper lip: a case report. Med Oral Patol Oral Cir Bucal. 2007;12:E1–3.
Pires FR, Pringle GA, de Almeida OP, et al. Intra-oral minor salivary gland tumors: a clinicopathological study of 546 cases. Oral Oncol. 2007;43:463–70.
Pons VO, Almendros MN, Berini AL, et al. Minor salivary gland tumors: A clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal. 2008;13:E582–8.
Regezi JA, Lloyd RV, Zarbo RJ, et al. Minor salivary gland tumors. A histologic and immunohistochemical study. Cancer. 1985;55:108–15.
Rousseau A, Mock D, Dover DG, et al. Multiple canalicular adenomas: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:346–50.
Sarangapani K, McCarthy J. Multiple cystic adenomas of labial salivary glands. Br J Oral Surg. 1977;15:166–70.
Siqueira CS, Fernandes KS, Vivas AP, et al. Clinical and histological features of multifocal canalicular adenomas of the upper lip. Braz Dent J. 2014;24:542–6.
Sivolella S, Valente M, De BM, et al. Canalicular adenoma immunoprofile: a case report. Gerodontology 2013. doi:10.1111/ger.12039.
Smullin SE, Fielding AF, Susarla SM, et al. Canalicular adenoma of the palate: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:32–6.
Stramandinoli-Zanicotti RT, Cesa TS, Giustina JD, et al. Canalicular adenoma of the minor salivary gland in the upper lip: case report. J Oral Diagn. 2012;1:4–6.
Stefanini M, Diokno RM. Monomorphic adenoma of lip, canalicular variant. IMJ Ill Med J. 1980;158:388–9.
Suarez P, Hammond HL, Luna MA, et al. Palatal canalicular adenoma: report of 12 cases and review of the literature. Ann Diagn Pathol. 1998;2:224–8.
Triantafyllou A, Coulter P, Scott J. Phenotypes in canalicular adenoma of human minor salivary glands reflect the interplay of altered secretory product, absent neuro-effector relationships and the diversity of the microenvironment. Histopathology. 1999;35:502–16.
Tyralik D, Dzierwa-Gawron A, Rys J. Canalicular adenoma of the upper lip. Metachronous (multifocal) canalicular adenoma of the upper lip: a case report of an unusual finding. Pol J Pathol. 2013;64:71–4.
Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323–33.
Werder P, Altermatt HJ, Zbaren P, et al. Canalicular adenoma of a minor salivary gland on the palate: a case presentation. Quintessence Int. 2009;40:623–6.
Wiener AP, Meadows F. Monomorphic adenoma, canalicular variant: report of case. J Oral Surg. 1977;35:414–5.
Yamada H, Ishii H, Seto K, et al. Canalicular adenoma of the buccal mucosa: a case report with computed tomography and magnetic resonance imaging. J Oral Maxillofac Surg. 2003;61:837–40.
Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg. 2005;63:805–10.
Yoon AJ, Beller DE, Woo VL, et al. Bilateral canalicular adenomas of the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:341–3.
Yuce S, Uysal IO, Dogan M, et al. Canalicular adenoma of the palate. J Craniofac Surg. 2012;23:e396–8.
Zohar Y, Shem-Tov Y, Gal R. Salivary duct carcinoma in major and minor salivary glands. A clinicopathological analysis of four cases. J Craniomaxillofac Surg. 1988;16:320–3.
Crumpler C, Scharfenberg JC, Reed RJ. Monomorphic adenomas of salivary glands. Trabecular-tubular, canalicular, and basaloid variants. Cancer. 1976;38:193–200.
van der Wal JE, Snow GB, van dW, I. Histological reclassification of 101 intraoral salivary gland tumours (new WHO classification). J Clin Pathol. 1992;45:834–5.
Chau MN, Radden BG. Intra-oral salivary gland neoplasms: a retrospective study of 98 cases. J Oral Pathol. 1986;15:339–42.
Mintz GA, Abrams AM, Melrose RJ. Monomorphic adenomas of the major and minor salivary glands. Report of twenty-one cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1982;53:375–86.
Chaudhry AP, Labay GR, Yamane GM, et al. Clinico-pathologic and histogenetic study of 189 intraoral minor salivary gland tumors. J Oral Med. 1984;39:58–78.
Rossiello R, Rossiello L, De SS, et al. Canalicular adenoma of the parotid gland: a case report. Anticancer Res. 2003;23:4101–3.
Lima SS, Soares AF, de Amorim RF, et al. Epidemiologic profile of salivary gland neoplasms: analysis of 245 cases. Braz J Otorhinolaryngol. 2005;71:335–40.
Jaber MA. Intraoral minor salivary gland tumors: a review of 75 cases in a Libyan population. Int J Oral Maxillofac Surg. 2006;35:150–4.
Ito FA, Ito K, Vargas PA, et al. Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;34:533–6.
Woo VL, Bhuiya T, Kelsch R. Assessment of CD43 expression in adenoid cystic carcinomas, polymorphous low-grade adenocarcinomas, and monomorphic adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:495–500.
Ansari MH. Salivary gland tumors in an Iranian population: a retrospective study of 130 cases. J Oral Maxillofac Surg. 2007;65:2187–94.
Muenscher A, Diegel T, Jaehne M, et al. Benign and malignant salivary gland diseases in children A retrospective study of 549 cases from the Salivary Gland Registry. Hamburg. Auris Nasus Larynx. 2009;36:326–31.
Tian Z, Li L, Wang L, et al. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg. 2010;39:235–42.
Curran AE, Allen CM, Beck FM, et al. Distinctive pattern of glial fibrillary acidic protein immunoreactivity useful in distinguishing fragmented pleomorphic adenoma, canalicular adenoma and polymorphous low grade adenocarcinoma of minor salivary glands. Head Neck Pathol. 2007;1:27–32.
Butler C, Kulendra KN, Menon G, et al. Canalicular adenoma: a case report of an unusual parotid lesion. BMJ Case. Rep. 2009. doi:10.1136/bcr.10.2008.1072.
Conceicao Barros A, Silva Gurgel CA, Caymmi Gomes M, et al. Minor salivary gland tumors in a South American population. Arch Oncol. 2010;18:56–9.
van der Wal JE, Leverstein H, Snow GB, et al. Parotid gland tumors: histologic reevaluation and reclassification of 478 cases. Head Neck. 1998;20:204–7.
Rotellini M, Palomba A, Baroni G, et al. Diagnostic Utility of PLAG1 Immunohistochemical Determination in Salivary Gland Tumors. Morphol: Appl Immunohistochem Mol; 2013.
Liess BD, Lane RV, Frazier S, et al. Bilateral canalicular adenoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 2006;132:339–41.
Etit D, Ekinci N, Tan A, et al. An analysis of salivary gland neoplasms: a 12-year, single-institution experience in Turkey. Ear Nose Throat J. 2012;91:125–9.
Zarbo RJ, Prasad AR, Regezi JA, et al. Salivary gland basal cell and canalicular adenomas: immunohistochemical demonstration of myoepithelial cell participation and morphogenetic considerations. Arch Pathol Lab Med. 2000;124:401–5.
Prasad AR, Savera AT, Gown AM, et al. The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med. 1999;123:801–6.
Manjunatha BS, Kumar GS, Raghunath V. Immunohistochemical expression of Bcl-2 in benign and malignant salivary gland tumors. Med Oral Patol Oral Cir Bucal. 2011;16:e503–7.
Prabhu S, Kaveri H, Rekha K. Benign, malignant salivary gland tumors: comparison of immunohistochemical expression of e-cadherin. Oral Oncol. 2009;45:594–9.
Gardner DG, Daley TD. The use of the terms monomorphic adenoma, basal cell adenoma, and canalicular adenoma as applied to salivary gland tumors. Oral Surg Oral Med Oral Pathol. 1983;56:608–15.
Dardick I, Rippstein P, Skimming L, et al. Immunohistochemistry and ultrastructure of myoepithelium and modified myoepithelium of the ducts of human major salivary glands: histogenetic implications for salivary gland tumors. Oral Surg Oral Med Oral Pathol. 1987;64:703–15.
Eversole LR. Histogenetic classification of salivary tumors. Arch Pathol. 1971;92:433–43.
Youngberg G, Rao MS. Ultrastructural features of monomorphic adenoma of the parotid gland. Oral Surg Oral Med Oral Pathol. 1979;47:458–61.
Tandler B, Denning CR, Mandel ID, et al. Ultrastructure of human labial salivary glands. 3. Myoepithelium and ducts. J Morphol. 1970;130:227–45.
Weinreb I, Simpson RH, Skalova A, et al. Ductal adenomas of salivary gland showing features of striated duct differentiation (‘striated duct adenoma’): a report of six cases. Histopathology. 2010;57:707–15.
Chenevert J, Duvvuri U, Chiosea S, et al. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919–29.
Ohtomo R, Mori T, Shibata S, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041–50.
Pires FR, de Almeida OP, Pringle G, et al. Differences on clinicopathological profile from intraoral minor salivary gland tumors around the world. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:136–8.
Philpott CM, Kendall C, Murty GE. Canalicular adenoma of the parotid gland. J Laryngol Otol. 2005;119:59–60.
Przewozny T, Stankiewicz C. Neoplasms of the parotid gland in northern Poland, 1991–2000: an epidemiologic study. Eur Arch Otorhinolaryngol. 2004;261:369–75.
Lim LH, Chao SS, Goh CH, et al. Parotid gland surgery: 4-year review of 118 cases in an Asian population. Head Neck. 2003;25:543–8.
Badoual C, Rousseau A, Heudes D, et al. Evaluation of frozen section diagnosis in 721 parotid gland lesions. Histopathology. 2006;49:538–40.
Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol. 2012;25:405–15.
Epivatianos A, Harrison JD. The presence of microcalculi in normal human submandibular and parotid salivary glands. Arch Oral Biol. 1989;34:261–5.
de Araujo V, de Sousa SO, Carvalho YR, et al. Application of immunohistochemistry to the diagnosis of salivary gland tumors. Appl Immunohistochem Mol Morphol. 2000;8:195–202.
Huang JW, Ming Z, Shrestha P, et al. Immunohistochemical evaluation of the Ca(2+)-binding S-100 proteins S-100A1, S-100A2, S-100A4, S-100A6 and S-100B in salivary gland tumors. J Oral Pathol Med. 1996;25:547–55.
Zarbo RJ, Regezi JA, Batsakis JG. S-100 protein in salivary gland tumors: an immunohistochemical study of 129 cases. Head Neck Surg. 1986;8:268–75.
Bilal H, Handra-Luca A, Bertrand JC, et al. P63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem. 2003;51:133–9.
Edwards PC, Bhuiya T, Kelsch RD. Assessment of p63 expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and basal cell and canalicular adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:613–9.
Weber A, Langhanki L, Schutz A, et al. Expression profiles of p53, p63, and p73 in benign salivary gland tumors. Virchows Arch. 2002;441:428–36.
Dhillon PK, Barry M, Stampfer MJ, et al. Aberrant cytoplasmic expression of p63 and prostate cancer mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:595–600.
Martin SE, Temm CJ, Goheen MP, et al. Cytoplasmic p63 immunohistochemistry is a useful marker for muscle differentiation: an immunohistochemical and immunoelectron microscopic study. Mod Pathol. 2011;24:1320–6.
Edwards PC, Bhuiya T, Kelsch RD. C-kit expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and monomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:586–93.
Stokes M, Foss R, Williams S. Reticular myoepithelioma: a Clinicopathologic correlation study (Poster Abstract Only). Oral Surg Oral Med. Oral Pathol. 2005;100:192.
Takeda Y, Kuroda M, Suzuki A. Ameloblastoma of mucosal origin. Acta Pathol Jpn. 1988;38:1053–60.
McFarland J. The histolopathologic prognosis of salivary sland mixed tumors. Am J Med Sci. 1942;203:502–19.
Ash JE. Mixed tumors of the salivary gland type: Preliminary report. Am J Orthod Oral Surg. 1947;33:522–31.
Bauer WH, Bauer JD. Classification of glandular tumors of salivary glands. Arch Pathol. 1953;55:328–48.
Bhaskar SN, Weinmann JP. Tumors of the minor salivary glands. Oral Surg. 1955;8:1278–97.
Calhoun NR, Cerine FC, Mathews MJ. Papillary cystadenoma of the upper lip. Report of a case. Oral Surg Oral Med Oral Pathol. 1965;20:810–3.
Rauch S, Seifert G, Gorlin RJ. Diseases of the salivary glands: tumors. In: Gorlin RJ, Goldman HM. Thomas oral pathology, 6th ed. St. Louis: C.V. Mosby Company, 1970.
Batsakis JG, Brannon RB, Sciubba JJ. Monomorphic adenomas of major salivary glands: a histologic study of 96 tumors. Clin Otolaryngol. 1981;6:129–43.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group. A special thank you to Ms. Hannah B. Herrera for her research assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, L.D.R., Bauer, J.L., Chiosea, S. et al. Canalicular Adenoma: A Clinicopathologic and Immunohistochemical Analysis of 67 Cases with a Review of the Literature. Head and Neck Pathol 9, 181–195 (2015). https://doi.org/10.1007/s12105-014-0560-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-014-0560-6