Abstract
The canonical Wnt/β-catenin pathway is involved in the formation of craniofacial skeleton and oral tissues. Aberrant nuclear localization of β-catenin protein has been described in several human diseases including a subset of odontogenic tumors thereby suggesting an important role in tumor development. Fibro-osseous lesions of the craniofacial skeleton comprise several neoplastic, and reactive mesenchymal proliferations in which β-catenin status is unknown. To study this, we immunostained 171 fibro-osseous lesions for β-catenin protein and, for lesions with nuclear positivity, sequenced exon 3 of the CTNNB1 gene and exon 15 of the APC gene. Nuclear β-catenin immunostaining was detected in 34 (20 %) tumors with no correlation between nuclear positivity and either age, gender, or tissue decalcification status (p = 0.2, 0.17, 0.12, respectively). Absent nuclear β-catenin in fibrous dysplasia was the only diagnostically significant finding (p = 0.0034). A single point mutation at Asp56 of CTNNB1 was identified in one case of ossifying fibroma. A second ossifying fibroma and one desmoplastic fibroma demonstrated point mutations (Glu1229 and Tyr1475, respectively) in the APC gene. These findings show that apart from fibrous dysplasia where nuclear β-catenin is rare, nuclear β-catenin staining has limited utility in discriminating among the craniofacial fibro-osseous lesions. The molecular mechanisms underlying nuclear β-catenin accumulation in the positive tumors is unlikely to be mediated by CTNNB1 exon 3 or APC exon 15 mutations in most cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In normal cells, the canonical Wnt signaling pathway regulates cell proliferation, differentiation and function by binding of Wnt to Frizzled and lipoprotein-related protein 5 or 6 receptors and downstream activation of β-catenin [1]. Whereas the Wnt/β-catenin pathway plays a critical role in the regulation of bone development and homeostasis, abnormal levels of β-catenin lead to a variety of skeletal disorders [2–7]. Somatic mutations in components of the Wnt/β-catenin pathway have been implicated in a variety of human neoplasms [8–11]. Specifically, mutations in the CTNNB1 gene, which encodes β-catenin, or the APC gene, lead to release of β-catenin from a membrane-bound complex, allowing it to accumulate in the nucleus and induce target gene expression. For example, desmoid-type fibromatosis can result from germ-line mutations in APC or somatic mutations in CTNNB1 [12–14]. The abnormal nuclear localization of β-catenin can also be used in diagnostic pathology to differentiate desmoid tumors from morphologic mimics [15]. The Wnt pathway also plays a role in the formation of the craniofacial skeleton [1, 16] and recent data suggest that β-catenin mutations are present in a subset of odontogenic tumors of the jaws [17, 18].
The jaws, cranium and facial bones are common sites for a group of conditions termed “fibro-osseous lesions”—mesenchymal proliferations characterized by abundant hypercellular fibrous tissue as well as ossification [19–23]. This disease category includes a diverse range of entities that lie in a continuum of reactive lesions (e.g. proliferative periostitis), developmental dysplasias (e.g. fibrous dysplasia, cemento-osseous dysplasia) and true neoplasms (ossifying fibroma). Some of these appear to be relatively specific to the craniofacial complex whereas others are distributed throughout the skeleton. [19, 24, 25] Although fibro-osseous lesions are largely benign, a low-grade osteosarcoma can also enter into the microscopic differential diagnosis. Finally, desmoplastic fibroma, though defined as a purely fibrous tumor of bone, can nevertheless contain a component of reactive or metaplastic bone and may be difficult to differentiate from other fibro-osseous lesions. The fibro-osseous lesions share significant morphologic overlap especially on limited samples such that definitive diagnosis can only be rendered in the context of clinical and radiographic findings. In practice, even an expert pathologist armed with histologic, clinical and radiographic data may have difficulty categorizing these lesions into specific types.
To date, little is known about the role of the Wnt/β-catenin pathway in fibro-osseous lesions of the craniofacial complex. Recent evidence implicates upregulation of Wnt/β-catenin signaling in fibrous dysplasia through Gαs protein modulation [26]. Desmoplastic fibroma is a possible candidate for aberrant β-catenin accumulation because of its morphologic similarity to desmoid fibromatosis of soft tissue. However, a recent study has failed to show nuclear accumulation of β-catenin or associated mutations in desmoplastic fibroma [25]. Although, it should be noted that the aforementioned study included very few craniofacial examples of desmoplastic fibroma.
Since alterations in β-catenin signaling, if any, among the heterogeneous group of craniofacial fibro-osseous lesions have not been determined, the objectives of this study were to: (1) define the prevalence of abnormal nuclear accumulation of β-catenin in a large number of fibro-osseous lesions of the craniofacial bones, (2) correlate β-catenin staining with APC and CTNNB1 mutation status, and (3) determine if β-catenin staining has diagnostic utility in this seemingly heterogeneous group of bone lesions.
Methods
Case Selection
We identified 171 fibro-osseous tumors of the jaw and cranial bones, in 163 patients, from the routine pathology and consultation files of the authors. The diagnoses were confirmed by review of histologic, radiographic and clinical variables based on standard diagnostic criteria [27]. Tumors with overlapping features between two distinct diagnostic entities, or in which consensus could not be reached between the authors, were classified as fibro-osseous lesions, not otherwise specified (NOS). Odontogenic tumors, low-grade osteosarcoma, osteomyelitis and aneurysmal bone cyst were excluded. The clinical records were searched for familial adenomatous polyposis (FAP), or syndromes associated with fibrous dysplasia (e.g. McCune–Albright).
Immunohistochemistry
Immunostaining was performed on formalin-fixed paraffin-embedded (FFPE) tissue. An attempt was made in every case to select a paraffin block that was not decalcified. If only decalcified tissue was available, this fact was recorded. Representative 4 μm sections were stained using the BD Biosciences (San Jose, CA) clone 14B β-catenin antibody (1:400) on a Leica (Buffalo Grove, IL) Bond III automated immunostainer. Sections were scored independently (by AEH and RJ,) blinded to the diagnoses, with consensus reached on all discrepant results. A result was considered positive if >10 % of tumor cells of both bone and fibrous tissue demonstrated strong nuclear staining. Cytoplasmic and membrane staining, if present, were scored as negative. Appropriate positive and negative controls were used.
DNA Isolation, PCR and Sequencing
Genomic DNA was extracted from FFPE sections using the QIAamp (QIAGEN, Valencia, CA) kit according to the manufacturer’s methods. CTNNB1 exon 3 and APC exon 15 were amplified in two and four separate fragments, respectively (PCR primers listed in Table 1), in order to maximize readable sequence from FPPE-tissue derived DNA. The PCR reactions consisted of 2.5 mM MgCl2, 0.1 mM dNTPs, 0.025 U of Platinum Taq polymerase (Invitrogen, Carlsbad, CA), 2 % DMSO, 1X PCR Buffer, 200 μM PCR primers and 10 ng of DNA template. A 2 μL reaction was run in the following conditions: 95 °C × 5 min, [94 °C × 20 s, 65 °C × 20 s (decreasing 0.5 °C per cycle), 72 °C × 45 s; 14 cycles], [94 °C × 20 s, 58 °C × 20 s, 72 °C × 45 s; 35 cycles], 72 °C for 10 min. The PCR products were treated with 0.5 U of SAP and 0.5 U of ExoI at 37 °C for 60 min, then inactivated at 90 °C for 15 min.
The PCR products were used as templates for the sequencing reaction with BigDye Terminator (Applied Biosystems, Foster City, CA) and the amplification primers. The sequencing reaction was cleaned up with X-Terminator (Applied Biosystems) and analyzed on the 3730xl DNA Analyzer (Applied Biosystems). The resulting sequencing data are viewed through Sequencher (GeneCodes, Ann Arbor, MI) to genotype for variations.
Statistical Analysis
Fisher’s exact test with Bonferroni correction for multiple comparisons was used to test for associations between β-catenin nuclear staining and diagnosis, clinical parameters and tissue decalcification status.
Results
Patient characteristics are summarized in Table 2. A total of 171 tumors from 163 patients (median age = 32 years, range 2–69; 48 male, 115 female) were included. No patients had documented FAP but two patients had syndromic fibrous dysplasia (one McCune–Albright, one with polyostotic fibrous dysplasia without myxomas or precocious puberty).
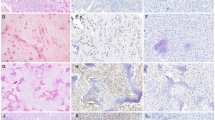
Nuclear β-catenin immunostaining (Fig. 1) was detected in 34 (20 %) tumors. In positive tumors, the staining was predominantly in the spindle cells occupying stroma between seams of osteoid, bone or cementum. Conversely, cells rimming or entrapped in matrix showed only membrane staining or were negative for β-catenin. There was no correlation between nuclear staining and patient age, gender, or tissue decalcification status (p = 0.2, 0.17, 0.12, respectively). Staining by tumor type is summarized in Table 3. The only diagnostically significant finding was absence of nuclear β-catenin in the fibrous dysplasias (4 % positive) relative to the other diagnoses where staining was more common (15–57 %; p = 0.0034). Among the fibro-osseous lesion NOS category, no consistent morphologic difference was noted between β-catenin positive compared to negative cases. In patients who had multiple tumors (either multifocal disease or primary and recurrence), separately tested for β-catenin, staining was concordant with the exception of one case: a mandibular cemento-osseous dysplasia in a 30-year old woman that was positive on initial resection, but negative in a recurrence 3 years later.
Representative histologic findings and nuclear β-catenin immunopositivity in fibro-osseous lesions. The majority of fibrous dysplasias (a, b) were negative for nuclear staining although membrane staining in tumor cells was frequently present. Desmoplastic fibroma (c, d), cemento-osseous dysplasia (e, f), ossifying fibroma (g, h), juvenile trabecular ossifying fibroma (i, j) more frequently demonstrated nuclear staining. An example of a fibro-osseous lesion, NOS that was also positive (k, l). This example contained areas similar to cemento-osseous dysplasia (shown) but other areas mimicked fibrous dysplasia and ossifying fibroma
Of the 34 β-catenin positive tumors, sequence data was interpretable in 23 tumors of which 15 contained single base variants. In CTNNB1 exon 3, only a single base substitution was identified, a T-to-C transition, in an ossifying fibroma, that corresponded to the silent third base in the Asp56 codon. In APC exon 15, one ossifying fibroma contained a G-to-C transversion resulting in a predicted Glu1317 → Gln amino acid change. One desmoplastic fibroma contained a G-to-A transition resulting in a premature stop codon at Asp1536. Silent G-to-A transitions at the third base of Tyr1493 in the APC gene were identified in an additional 12 tumors. DNA from the remaining 11 lesions (5 fibro-osseous lesion NOS, 3 desmoplastic fibroma, 2 ossifying fibroma, 1 cemento-osseous dysplasia) could not be successfully PCR amplified despite repeated attempts.
Discussion
In the present study, we demonstrate that nuclear β-catenin staining is present in a subset (20 %) of a diverse group of fibro-osseous lesions. The findings are in keeping with the wide range of soft tissue and bone tumors that can show at least low-level nuclear β-catenin positivity [14]. However, this nuclear β-catenin expression lacks the specificity to discriminate among the fibro-osseous lesions. The only exception to this finding is the relative absence of β-catenin staining in fibrous dysplasias (4 %) compared to the other lesions in which nuclear staining was more common. Thus, positive nuclear β-catenin staining may be useful to help exclude fibrous dysplasia when considering the differential diagnosis of fibro-osseous lesions of the craniofacial bones. The relative absence of β-catenin accumulation in fibrous dysplasia, including in two examples from polyostotic forms was unexpected in light of the recent finding that abnormal Gαs proteins in fibrous dysplasia upregulate β-catenin signaling [26]. The reason for this discrepancy is unknown but may stem from what specifically is interpreted as a positive result. Although we did note cytoplasmic β-catenin staining in some stromal cells and reactive osteoblasts in cases of fibrous dysplasia, similar to the findings of Regard et al. [26], we only scored as positive those lesions with unequivocal nuclear staining to remain consistent with prior methods using this marker [13–15, 25, 28–30]. The difference may also derive from divergent pathogenic mechanisms between craniofacial, as opposed to long bone, fibrous dysplasias. Alternatively, there may be temporal changes in nuclear β-catenin accumulation in fibrous dysplasia as the lesion progresses and craniofacial tumors, given their superficial and cosmetically obvious location, may come to clinical attention sooner. Interestingly, we do note that the two β–catenin positive fibrous dysplasias in our series were from patients in their late 20 s whereas the majority of the remaining cases presented in their early teens. We noted no morphologic differences between β-catenin positive and negative fibrous dysplasias.
A number of neoplasms have been shown to harbor somatic CTNNB1 or APC gene mutations leading to upregulation of the Wnt/β-catenin pathway. For example, sporadic desmoid tumors contain CTNNB1 exon 3 mutations involving codons 33, 34, 37, 41 or 45 [31]. By contrast, mutations in exon 15 of the APC gene can be seen in familial forms of desmoid tumors and other neoplasms [10, 32]. Mutations in this region may result in a truncated APC protein that resists proteasomal degradation [33]. In the present study, despite nuclear β-catenin expression, there were no missense or nonsense mutations in exon 3 of CTNNB1. In exon 15 of the APC gene, we identified two non-synonymous mutations, one of which would result in a predicted truncation of the protein at amino acid 1535 in a desmoplastic fibroma. However, the majority of lesions with nuclear β-catenin staining were either wild-type or harbored only silent mutations. Combined with the fact that none of the patients with β-catenin positive tumors had a history of FAP, it is not likely that β-catenin overexpression and nuclear localization are due to APC exon 3 or CTNNB1 exon 15 mutation in fibro-osseous lesions of the craniofacial bones.
A limitation of this study is that we were unable to obtain meaningful sequence data from all lesions with nuclear β-catenin staining. Although we did demonstrate that decalcification did not significantly affect immunohistochemistry it is possible that processing techniques, especially decalcification, deteriorated DNA quality. We attempted to minimize the effects of highly fragmented DNA by designing primers to produce relatively short amplicons. Nevertheless, in five (46 %) of the non-informative cases, only acid-decalcified tissue was available, which is known to affect DNA quality [34]. The distribution of diagnoses is approximately the same in the lesions which yielded meaningful sequence as in the group which did not, so the limitation is not likely to affect our conclusions.
As in prior studies, we sequenced only exon 3 and exon 15 of CTNNB1 and APC genes respectively [13, 14, 17, 25, 30, 35]. Although most studies suggest that tumor-promoting mutations map to these two exons, we cannot exclude the possibility that mutations in other exons, or in noncoding regions, may contribute to the aberrant nuclear accumulation of β-catenin in fibro-osseous lesions of the craniofacial skeleton. Whether the immunohistochemical expression of β-catenin in craniofacial fibro-osseous lesions is driven by such mutations, by epigenetic mechanisms or by alterations in other steps in the Wnt/β-catenin pathway remains to be determined.
In summary, nuclear staining for β-catenin is present in fibro-osseous lesions of the craniofacial skeleton implicating the Wnt/β-catenin pathway in the pathogenesis of a subset of these lesions. With the exception of fibrous dysplasia, in which nuclear staining is rare, the remaining categories of fibro-osseous lesions show similar incidences of nuclear β-catenin positivity. Thus, the finding of nuclear β-catenin immunostaining has limited utility in discriminating among the other entities. The molecular mechanisms underlying nuclear β-catenin accumulation in the positive tumors are unlikely to be mediated by CTNNB1 exon 3 or APC exon 15 gene mutations in most cases.
References
Liu F, Millar SE. Wnt/β-catenin signaling in oral tissue development and disease. J Dent Res. 2011;89(4):318–30.
Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–50.
Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am. 2008;90(Suppl 1):19–24.
Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–23.
Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–38.
Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. 2004;101(26):9757–62.
Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44.
Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92(7):3046–50.
Palacios J, Gamallo C. Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58(7):1344–7.
Abutaily AS, Collins JE, Roche WR. Cadherins, catenins and APC in pleural malignant mesothelioma. J Pathol. 2003;201(3):355–62.
Iwai S, Katagiri W, Kong C, Amekawa S, Nakazawa M, Yura Y. Mutations of the APC, β-catenin, and axin 1 genes and cytoplasmic accumulation of β-catenin in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131(12):773–82.
Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased β-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol. 1997;151(2):329–34.
Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, Ricci F, Weber K, Furlong MA, et al. Nuclear β-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol. 2005;29(5):653–9.
Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, et al. Nuclear β-catenin in mesenchymal tumors. Mod Pathol. 2005;18(1):68–74.
Carlson JW, Fletcher CD. Immunohistochemistry for β-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007;51(4):509–14.
Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc Natl Acad Sci USA. 2006;103(49):18627–32.
Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, et al. β-Catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol. 2003;163(5):1707–12.
Siriwardena BS, Kudo Y, Ogawa I, Tilakaratne WM, Takata T. Aberrant β-catenin expression and adenomatous polyposis coli gene mutation in ameloblastoma and odontogenic carcinoma. Oral Oncol. 2009;45(2):103–8.
Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2(3):177–202.
Stewart JCB. Benign, nonodontogenic tumors. In: Regezzi JA, Sciubba JJ, Jordan RC, editors. Oral pathology clinical pathologic correlations. 6th ed. St. Louis: Elsevier; 2012. p. 293–302.
Slootweg PJ, El Mofty SK. Ossifying fibroma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. 1st ed. Lyon: IARC Press; 2005. p. 319–20.
Jundt G. Fibrous dysplasia. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. 1st ed. Lyon: IARC Press; 2005. p. 321–2.
Slootweg PJ. Osseous dysplasias. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. 1st ed. Lyon: IARC Press; 2005. p. 323.
Siegal GP, Bianco P, Dal Cin P. Fibrous dysplasia. In: Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. p. 352–3.
Hauben EI, Jundt G, Cleton-Jansen AM, Yavas A, Kroon HM, Van Marck E, et al. Desmoplastic fibroma of bone: an immunohistochemical study including β-catenin expression and mutational analysis for β-catenin. Hum Pathol. 2005;36(9):1025–30.
Regard JB, Cherman N, Palmer D, Kuznetsov SA, Celi FS, Guettier JM, et al. Wnt/β-catenin signaling is differentially regulated by Gα proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci USA. 2012;108(50):20101–6.
Barnes L, Eveson J, Reichart P, Sidransky D. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005.
Horvai AE, Kramer MJ, O’Donnell R. β-Catenin nuclear expression correlates with cyclin D1 expression in primary and metastatic synovial sarcoma: a tissue microarray study. Arch Pathol Lab Med. 2006;130(6):792–8.
Hasegawa T, Yokoyama R, Matsuno Y, Shimoda T, Hirohashi S. Prognostic significance of histologic grade and nuclear expression of β-catenin in synovial sarcoma. Hum Pathol. 2001;32(3):257–63.
Saito T, Oda Y, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, et al. β-Catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumours. J Pathol. 2001;195(2):222–8.
Le Guellec S, Soubeyran I, Rochaix P, Filleron T, Neuville A, Hostein I, et al. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: a study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol. 2012;25(12):1551–8.
Kotiligam D, Lazar AJ, Pollock RE, Lev D. Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol. 2008;23(1):117–26.
Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001;10(7):721–33.
Singh VM, Salunga RC, Huang VJ, Tran Y, Erlander M, Plumlee P, et al. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann Diagn Pathol. 2013;17(4):322–6.
Kondo Y, Kanai Y, Sakamoto M, Genda T, Mizokami M, Ueda R, et al. β-catenin accumulation and mutation of exon 3 of the β-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999;90(12):1301–9.
Acknowledgments
We would like to thank Wendy Chan B. S. and Jon Woo M. S. of the UCSF Genomics Core Facility for their expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
E. Horvai, A., C. Jordan, R. Fibro-Osseous Lesions of the Craniofacial Bones: β-Catenin Immunohistochemical Analysis and CTNNB1 and APC Mutation Analysis. Head and Neck Pathol 8, 291–297 (2014). https://doi.org/10.1007/s12105-014-0535-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-014-0535-7