Abstract
Cribriform adenocarcinoma of the tongue and minor salivary glands (CATMSG) is a tumor occurring mostly, but not exclusively, in the base of the tongue. Other locations are minor salivary glands of the oral cavity. Histopathologically, CATMSG resembles papillary carcinoma of the thyroid gland. It usually reveals a solid growth devoid of colloid, and eosinophilic material present in follicular areas is rather pale in contrast to metastatic foci seen in papillary thyroid carcinoma that shows typical deeply eosinophilic colloid with “moth-eaten peripheries” and cystic configuration. In addition, giant multinucleated cells are not observed in CATMSG and psammoma bodies are found only exceptionally. Unlike papillary thyroid carcinoma, CATMSG is composed of hybrid secretory-myoepithelial cells. Most importantly, CATMSG is consistently negative with both thyroglobulin and TTF-1. CATMSG is a distinct tumor entity that also differs from polymorphous low-grade adenocarcinoma by location, cytology, histological architecture, and behavior, with frequent metastases at the time of presentation. Paradoxically, early metastatic disease seen in most cases of CATMSG is associated with an indolent behavior. It makes CATMSG a unique neoplasm among all low-grade salivary gland tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cribriform adenocarcinoma of the tongue and minor salivary glands (CATMSG) is the tumor originally described in 1999 by Michal et al. [1] under the name cribriform adenocarcinoma of the tongue. In the original series, all 8 neoplasms were located in the tongue and their morphology was similar to papillary carcinoma of the thyroid gland resulting in a presumption that cribriform adenocarcinoma may have an origin in the lingual thyroglossal duct anlage [1]. Prior to that publication, one of the authors (M.M) had been aware of the existence of this interesting neoplasm since the late 1980s when he received three cases over a short span of time. The cases were filed as “strange poorly differentiated synovial sarcoma-like tumors” in our registry. After the original paper was published 1999 [1], we started to receive identical tumors located outside the tongue, including the soft palate, retromolar buccal mucosa and lip which seriously questioned the theory of the origin in the lingual thyroglossal duct anlage. This new information was summarized in a recent paper published by our group, and cribriform adenocarcinoma of the tongue was renamed as cribriform adenocarcinoma of minor salivary glands [2].
According to the current WHO Classification of Tumours of the Head and Neck, CATMSG is a provisional entity without a clear statement as to whether it represents a genuine entity or is merely a variant of polymorphous low-grade adenocarcinoma (PLGA) [3]. We and others are convinced, however, that CATMSG is a distinct neoplasm, and that it should be considered separately from PLGA [4–8].
Clinical, Gross and Histopathological Findings
Of the 31 published cases in the literature [2–7], 21 tumors were located in the tongue (usually the base), 3 in the soft palate, 2 in the retromolar buccal mucosa, 3 in the lingual tonsils, 1 in the upper lip and 1 in the floor of the mouth. One tumor located in the tongue was described to have a pedunculated configuration [4]. The sex was known in 27 cases: the tumors occurred in 15 women and 12 men. The age of the patients ranged from 21 to 85 (mean 56.8 years). The cervical lymph node metastases were present in the lateral neck at the time of diagnosis in 19/31 of the patients. Three neck lymph node metastases were bilateral. The tumors were treated by surgical excision often accompanied by neck lymph node dissection. Of the 31 patients, 14 individuals additionally received radiotherapy, and 1 adjuvant chemotherapy. Clinical follow-up was known in 21 cases. All patients with available follow-up (range 2 months to 13 years; mean 4.3 years) were alive and without signs of metastases.
Grossly, the tumors are unencapsulated, white-tan to grey in color, and hard in consistency with no areas of necrosis and hemorrhage.
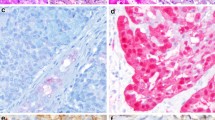
Histopathologically, the tumor is almost always covered by an intact squamous epithelium devoid of ulceration or dysplasia (Fig. 1). Each tumor has invasive margins and in most cases infiltrates the muscular layer of the tongue and/or adjacent tissues. Lymphovascular invasion is observed in a third of cases. The tumors themselves are composed predominantly of cribriform (Fig. 2a) to microcribriform (Fig. 2b) and solid structures (Fig. 2c) in variable proportions. In most instances, the tumor architecture consists mainly of a solid mass, often divided by fibrous septa into irregularly shaped and sized nodules composed of centrally necrotic areas (Fig. 1). In the solid areas, the tumor nests are frequently detached from the surrounding fibrous stroma by (presumably artifactual) clefts (Fig. 3), often resulting in a glomeruloid appearance (Fig. 3). The peripheral layer of such solid tumor nests often displays hyperchromatic nuclei in a somewhat palisaded pattern (Fig. 4a). Rarely, the central parts of tumor lobules reveal a clear cell change, which accentuate the peripheral palisading (Fig. 4b). In rare cases (2/28), psammoma bodies are found (Fig. 5). Typically, the tumors also include an intermingled tubular pattern. The tubules are approximately all of the same size and they are formed by a single cell layer (Fig. 5). A characteristic feature seen in more than a third of cases in our series was presence of mucinous-spindle cell myofibroblastic stromal septa (a previously unpublished feature), composed of mucinous matrix and rare spindle cell myofibroblasts, seen mostly in early infiltrative foci (Fig. 6). The mucin in the stroma has probably originated from the ruptured glands (Fig. 6).
Left CATMSG is practically always covered by an intact squamous epithelium devoid of ulceration and dysplasias. Right in most instances, the tumor architecture consist mainly of a solid mass, often divided by fibrous septa into irregularly shaped and sized nodules composed of centrally necrotic areas
The most prominent feature of the tumors, however, is the appearance of the nuclei. These often overlap one another, and are pale (Fig. 7a), optically clear and vesicular with a ground glass appearance (Fig. 7b). Rarely, there are solid areas composed of these cells with optically clear nuclei (Fig. 7c). Cellular atypia is mild, and mitotic figures are in most cases rare. Generally, there are one to three small inconspicuous nucleoli. The cytoplasm is clear to eosinophilic. Cytologically, all the tumors are composed of one cell type. The overall morphology of the tumor, particularly with focal papillary growth and with overlapping clear “Orphan Annie eye–like nuclei”, is remarkably similar to various variants of papillary thyroid carcinoma. The cervical lymph node metastases have usually identical appearances to the primary tumors.
Ultrastructure
Ultrastructurally, all the cells of the tumor are uniform and are thus of one type. They have irregularly clefted nuclei with nucleoli. The cytoplasm contains few organelles, including small numbers of mitochondria, lysosomes, and Golgi apparatus. Rare cells contain stacks of confronting cisternae of rough endoplasmic reticulum. The cells are attached one to each other by well formed desmosomes. Interestingly, at the ultrastructural level, even the areas with a solid appearance at the light microscopical level (Fig. 2c) reveal a microcribriform arrangement. The secretory spaces are composed of well-formed microvilli on the apical borders of the cells. A further unusual feature is that many of the secretory cells displaying the apical microvilli also contain groups of microfilaments in the cytoplasm (Fig. 8). These cells thus had features of hybrid myoepithelial-secretory cells, and had thus all aspects of “secretory myoepithelia”. Many cells, especially those in areas, which showed spindling of neoplastic cells, are found to contain numerous bundles of cytoplasmic tonofilaments [1, 2]. Similar hybrid secretory myoepithelia have recently been described in the breast by Del Vecchio et al. [9], who reported a cell type with hybrid secretory and myoepithelial differentiation in three cases of lobular carcinoma, two of which had an infiltrating component. These cells displayed not only the typical lobular cytomorphology, but also myoepithelial differentiation as shown by several myoepithelial markers. Although there are reports of additional mammary adenocarcinomas exhibiting similar hybrid secretory and myoepithelial differentiation [10–12] the presence of these cells in CATMSG has never been described in other salivary gland neoplasms and seems to be unique.
Immunohistochemical Findings
CATMSGc react strongly with antibodies to cytokeratin markers AE1-3, CAM5.2, CK7, CK8, CK18 as well as S-100 protein, smooth muscle actin (Fig. 8), calponin and vimentin. In addition, a significant positivity for c-kit (CD117) in 10–80 % cells with strong cytoplasmic and membranous expression has been reported. Variable positivity in 5–60 % of the cells is observed with antibody p16. Immunostaining for cyclin D1 and p53 protein demonstrate variable percentages of positive nuclei ranging between 0 and 35 % (mean 15 %) and 0–10 % (mean 2.6 %), respectively [2]. Basal and myoepithelial cell markers, such as p63, calponin, CK14, and CK5/6 are positive in all tumors with variable proportions up to 60 % often at the periphery of the tumor nodules, especially marking the palisaded cells surrounding the glomeruloid structures. Expression of CK19 was variable with mild to moderate staining of membranes and cytoplasm in 12 out of 17 cases in two studies [2, 7]. EMA, EGFR, HER-2/neu, ER, and PR are usually negative. More importantly all the tumors are invariably completely devoid of any staining for TTF-1 and thyroglobulin. The Ki-67 proliferation index was generally low [2, 7].
Molecular Findings
No somatic mutations of BRAF, K-RAS, H-RAS, N-RAS, c-kit, and PDGFRa genes were found in any of the analyzable cases in two papers [2, 7]. However, in RET proto-oncogene, heterozygous polymorphism Gly691Ser in exon 11 (1 case), heterozygous polymorphism p.Leu769Leu in exon 13 (1 case), heterozygous polymorphism Ser904Ser in exon 15 (1 case), and intronic variant p.IVS14-24 G/A of exon 14 (2 cases) were found in one study [7]. In 1 of the 15 cases studied, high-risk HPV type 33 was detected. In addition, the same case showed a weak positivity of HPV18 [2, 7]. This HPV positive case showed diffuse p16 positivity in 100 % of the tumor cells [2]. The remaining 14 cases tested for HPV were negative [2, 7].
Differential Diagnosis
The most important differential diagnosis of CATMSG is PLGA. This neoplasm typically has a wide range of architectural appearances, including tubule and fascicle formation, as well as solid, cribriform and sometimes small papillary structures. A particularly characteristic feature of PLGA is the occurrence of streaming columns of single file or narrow trabeculae of cells forming concentric whorls, thereby creating a target-like appearance [3]; perineural invasion is often seen, but does not indicate more aggressive behavior. At the cellular level, PLGA quite often contains clear cells and less frequently, mucous cells. In 3–5 % of cases of PLGA, crystals resembling the tyrosine rich crystals of some pleomorphic adenomas can be found [13, 14]. None of these features have ever been described in CATMSG.
Perhaps most importantly, the most striking feature of CATMSG is the great nuclear similarity to papillary carcinoma of the thyroid, and this is not seen to any great extent in PLGA.
Further, PLGA only rarely metastasizes, and it is our view that a good proportion of the putative examples of metastasizing PLGA could probably represent CATMSG, which had been included in some published series of PLGA. The literature records several possible examples of CATMSG published as PLGA or under other names. Perez-Ordonez et al. [15] described 17 PLGAs among which there were two examples of “PLGA” of the tongue. In this series, there was a much higher metastatic rate than is usual for PLGA, as 5 of the 17 cases had secondaries in the neck lymph nodes. Two of these five cases had a papillary appearance, and Fig. 4a, b of this paper show a tumor with an appearance very similar if not identical to that of CATMSG [15]. It is reasonable to speculate that the tumor pictured in these two figures is likely to be a CATMSG. Another possible candidate of CATMSG was reported by Colmenero et al. [16]. In a series of 14 PLGAs, one tumor involved the base of the tongue and metastasized to the neck lymph nodes. Their Fig. 2 (and possibly also Fig. 3) shows a tumor very similar to CATMSG. Interestingly, Figure 55 of the 2nd edition of the WHO classification of salivary gland neoplasms issued in 1991 [17] illustrates, under the heading of PLGA, a tumor quite similar to those in our paper, which perhaps represents another example of CATMSG. Two other possible candidates for CATMSG were published under different names, and both carcinomas metastasized to the cervical lymph nodes. Yajima et al. [18] reported a tumor of the tongue in a 59-year old man which was called “tubular adenocarcinoma”, and Crocker et al. [19] described a 5-year-old boy with an adenocarcinoma, which the authors designated “papillary adenocarcinoma of minor salivary gland”. Even allowing for the difficulties of proper assessment of figures in reprints, there are many similarities to the microscopic appearance of our cases. Particularly reminiscent are the pallor and ground glass quality of the nuclei as seen in both tumors, the solid and cribriform arrangement in the first tumor [18] and the papillary appearance resulting from tumor cell detachment from the stroma as seen in the other report [19]. As a consequence therefore, we strongly suspect that if cases of CATMSG were to be excluded from the figures for PLGA, then the metastatic rate of true PLGA would be much lower. In contrast, CATMSG had lymph node metastases in 19/31 cases already at the time of the presentation of the primary tumor in the published series, which makes CATMSG unique among all low-grade salivary gland tumors.
Another important differential diagnosis, especially in cases where the primary tumor is occult and excision of a metastatic focus in a lateral neck lymph node is the first biopsy obtained from a patient, is a metastasis of a papillary carcinoma of thyroid gland. One illustrative case was described by Laco et al. [7]. In their case 4, the original CATMSG located in the mouth floor was misdiagnosed as “proliferating pleomorphic adenoma” and no further treatment was judged necessary. The lymph node lesion which developed 37 months after removal of the primary tumor was misdiagnosed as a metastasis of papillary thyroid carcinoma. Total thyreoidectomy was performed but no tumor was found. Subsequently, the lymph node was sent for a second opinion and the diagnosis of metastasizing CATMSG was established. Biopsies taken at that time from the nasopharynx, base of tongue and palatine tonsils were negative. Therefore, the original tumor was finally reviewed and diagnosed as CATMSG. Thus, the correct diagnosis of the primary tumor took 45 months and because of misdiagnosis of CATMSG as a metastatic thyroidal papillary carcinoma led to unnecessary thyroidectomy [7]. CATMSG usually reveals a solid growth devoid of colloid, and any eosinophilic material present in follicular areas is rather pale. In contrast, metastatic foci of papillary thyroid carcinoma are frequently cystic and typically show deeply eosinophilic colloid with ‘moth-eaten’ peripheries. In addition, giant multinucleated cells are not observed in CATMSG and psammoma bodies are found only exceptionally. Unlike papillary thyroid carcinoma, the CATMSG is composed of hybrid secretory-myoepithelial cells. Most importantly, CATMSG is consistently negative with both thyroglobulin and TTF-1 [1, 2, 4–7]. However, one must be alert to the fact that both CATMSG and papillary thyroid carcinoma may show variable expression of galectin-3, CK 19 and HBME-1 [7].
Conclusions
We believe that CATMSG is a distinct tumor entity that differs from PLGA by location (i.e. most often arising on the tongue), cytology represented by hybrid hybrid myoepithelial-secretory cells, histological architecture, and behavior, with frequent metastases at the time of presentation of the primary tumor. Early metastatic disease seen in most cases of CATMSG associated with indolent behavior makes it a unique neoplasm among all low-grade salivary gland tumors.
References
Michal M, Skálová A, Simpson RHW, Raslan WF, Curik R, Leivo I, Mukensnabl P. Cribriform adenocarcinoma of the tongue: a hitherto unrecognized type of adenocarcinoma characteristically occurring in the tongue. Histopathology. 1999;35:495–501.
Skalova A, Sima R, Kaspirkova-Nemcova J, Simpson RHW, Elmberger G, Leivo I, Di Palma S, Jirasek T, Gnepp DR, Weinreb I, Perez-Ordonez B, Mukensnabl P, Rychly B, Hrabal P, Michal M. Cribriform adenocarcinoma of minor salivary gland origin principally affecting the tongue: characterization of new entity. Am J Surg Pathol. 2011;35:1168–76.
Luna MA, Wenig BM. Polymorphous low-grade adenocarcinoma. In: Barnes EL, Eveson JW, Reichart P, et al., editors. World Health Organization Classification of Tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 223–4.
Prasad KC, Kaniyur V, Pai RR, Nesari SS. Pedunculated cribriform adenocarcinoma of the base of the tongue. Ear Nose Throat J. 2004;83:62–4.
Borowsky-Borowy P, Dyduch G, Papla B, Gabrys I, Skladzien J, Okon K. Cribriform adenocarcinoma of the tongue. Pol J Pathol. 2011;3:168–71.
Cocek A, Hronkova K, Voldanova J, Sach J, Skalova A, Ambrus M, Vranova J, Hahn A. Cribriform adenocarcinoma of the base of the tongue and low-grade, polymorphic adenocarcinomas of the salivary glands. Oncol Lett. 2011;2:135–8.
Laco J, Kamaradova K, Vitkova P, Sehnalkova E, Dvorakova S, Vaclavikova E, Sykorova V, Kaspirkova J, Skalova A, Ryska A. Cribriform adenocarcinoma of minor salivary glands may express galectin-3, cytokeratin 19, and HMBE-1 and contains polymorphisms of RET and H-RAS proto-oncogenes. Virchows Arch. 2012;461:531–40.
Luna M. Controversial salivary gland lesions. Pathology. 2005;97:61–4.
Del Vecchio M, Foschini MP, Peterse JL, Eusebi V. Lobular carcinoma of the breast with hybrid myoepithelial and secretory (“myosecretory”) cell differentiation. Am J Surg Pathol. 2005;29:1530–6.
Shousha S, Knee G. In-situ lobular/myoepithelial neoplasia of the breast. Histopathology. 2004;45:93–5.
Soares J, Tomasic G, Buciarelli E, Eusebi V. Intralobular growth of myoepithelial cell carcinoma of the breast. Virchows Arch. 1994;425:205–10.
Tamai M. Intraductal growth of malignant mammary myoepithelioma. Am J Surg Pathol. 1992;16:116–1125.
Raubenheimer EJ, Van Heerden WFP, Thein T. Tyrosine-rich crystalloids in a polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1990;70:480–2.
Cleveland DB, Cosgrove MM, Martin SE. Tyrosine-rich crystalloids in a fine needle aspirate of a polymorphous low grade adenocarcinoma of a minor salivary gland. Acta Cytol. 1994;38:247–51.
Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32:521–9.
Colmenero CM, Patron M, Burgueno M, Sierra I. Polymorphous low-grade adenocarcinoma of the oral cavity. A report of 14 cases. J Oral Maxillofac Surg. 1982;50:595–600.
Seifert G, Sobin LH. Histological classification of salivary gland tumours. 2nd ed. Berlin: Springer; 1991.
Yajima M, Yamazaki T, Minemura T, Kotani A. Tubular adenocarcinoma of the apex of the tongue. J Oral Maxillofac Surg. 1989;47:86–8.
Crocker TP, Kreutner A, Othersen HB, Garvin J. Papillary adenocarcinoma of minor salivary gland origin in a child. Arch Otolaryngol. 1983;109:827–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michal, M., Kacerovska, D. & Kazakov, D.V. Cribriform Adenocarcinoma of the Tongue and Minor Salivary Glands: A Review. Head and Neck Pathol 7 (Suppl 1), 3–11 (2013). https://doi.org/10.1007/s12105-013-0457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-013-0457-9