Abstract
Childhood tuberculosis does not get the attention it deserves, both in the general child health services and the TB specific services. The difficulty in identification of the organism due to lack of proper sample as well as lower sensitivity of the smear, made it harder to detect cases with ease in the community. Newer diagnostic methods like cartridge based nucleic acid amplification tests (CBNAAT) and line probe assays (LPA) have the capacity to rapidly identify Mycobacterium tuberculosis with an improved sensitivity over the smear testing and have been employed under Revised National Tuberculosis Control Programme (RNTCP) across the country. As the symptoms suggestive of TB are very common and overlapping, the final yield of TB testing is better if the microbiological confirmation is done on good quality specimen from cases suspected of TB based on clinical and radiological abnormalities. Newer tests also provide simultaneous detection of much critical Rifampicin resistance. A Rifampicin resistant case is not only unlikely to respond to first-line standard therapy but such a treatment can result in further amplification of resistance to other companion drugs. Prevention of spread of the drug resistant disease thus requires that the treatment is guided by universal drug sensitivity testing (U-DST) of all TB cases. Furthermore, several changes have come up in the treatment of TB and are discussed. The dosages of anti TB drugs have been revised upwardly for optimal drug levels and now the fixed drug combinations are used under RNTCP. With the awareness about high initial Isoniazid (INH) resistance and its contribution to failure of retreatment regime, a companion third drug (Ethambutol) has been added to the continuation phase of the first-line therapy. The standard retreatment regime, better known as category II therapy, has been replaced by specific therapy as per the resistance pattern detected. The TB control activities have thus evolved a lot and the present article discusses the evolution and the current status of diagnostics and therapy of TB in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
WHO has identified TB in children as an important area of intervention as the world takes up the formidable challenge of eliminating TB. A road map to end TB among children has been developed [1]. India has launched the ambitious programme to make India TB free by 2025 and this obviously cannot happen without a focussed strategy for children with TB. Most common childhood illnesses are dealt under child survival and child health programmes. Acute diarrheal and respiratory illnesses not only make high contribution to morbidity and mortality among children but are also easy to identify and have simple and effective community management tools thus making them a public health favourite. In contrast, TB in children is not recognised as a major killer though the impact of the disease can be far more devastating and disabling among children. Lack of an easy and reliable diagnostic tool, and, prolonged therapy often leads to poor recognition and acceptance of diagnosis of TB among the community of patients or their caregivers and care providers.
Revised National Tuberculosis Control Programme (RNTCP) manages and implements the national policy for tuberculosis control. The infectious adults with TB form the main focus of programme policy, strategies and interventions. Such cases can be easily identified by a point of care staining for Acid fast bacilli (AFB) of sputum, even at the periphery. However, the same test is far less sensitive and the access to specimen is so poor that this tool is not as good for children. TB in a child is not like TB in a miniature adult, for it has many important differences in presentation of the disease, type of the disease, diagnostic and care pathways. The public health strategies used for managing TB among adults are often not suited for children as the disease process and diagnostic pathways may be different e.g., inability to provide an easily testable specimen like spontaneously produced sputum; poor sensitivity of the commonly employed tests for diagnosis like smear for AFB, reliance on chest radiology, etc. Retro-fitting the strategies for children to the existing adult oriented programme template creates difficulties. A successful strategy to manage children with TB shall require to take into account all the specific nuances related to it.
Despite all these odds, in our country, RNTCP has made efforts to include childhood under its fold and has to its credit many initiatives like the very first joint guideline in 2004 with Indian Academy of Pediatrics (IAP) [2] which has subsequently been updated several times; introduction of pediatric patient wise boxes for treatment; and, more recently, introduction of upfront testing of pediatric samples by newer tests like cartridge based nucleic acid amplification tests (CBNAAT).
TB care community all over the world feels that there is a definite possibility of the transformation of the current epidemic of TB to the next epidemic of drug resistant TB, despite shrinking numbers of cases. Therefore, in recent times, innovative technologies with possibility of scaling up of capacities to rapidly detect drug resistance have come up due to a concerted global effort. The leaders of this rapid diagnostic pack are CBNAAT and line probe assays (LPA); and, efficient liquid culture methods like Mycobacterium growth indicator tube (MGIT™). Over past decade, RNTCP has absorbed these newer technologies in a scaled up fashion covering the whole country, developed better and larger laboratory network. It has, therefore, now aligned its strategy to upfront detection of drug resistance for an early management in order to prevent further spread of disease as well as amplification of resistance.
The present article provides an insight into how this evolved TB management scenario has impacted the childhood TB management; providing basis for some of the key changes that are being made for diagnosis as well as treatment among children.
Evolution of Diagnostic Tools under RNTCP
For Confirmation of Tuberculosis
Ziehl- Nelsen (ZN) staining of the sputum or gastric aspirate was the primary strategy for microbiological confirmation under RNTCP but was not adequately utilised as the smear positivity among children with paucibacillary primary disease was very low (around 10%). Further, lack of skills or facilities for collection and processing of non-sputum specimens at the designated microscopic centres further decreased the use of this diagnostic modality.
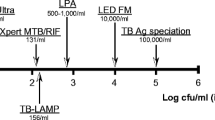
RNTCP approved newer tests like CBNAAT (e.g., Xpert-Rif™ from Cepheid, USA and TrueNat™ from MolBio, India) have provided an alternative point of care, rapid method for microbiological confirmation. These tests can provide result in a matter of about a couple of hours of testing time. Xpert Rif™ has been shown to have a much higher sensitivity than ZN smear as it requires just above a hundred DNA copies (organisms) per ml of the specimen as compared to tens of thousands for a smear. The studies have reported a 3-fold increase in the diagnostic yield with this tool [3]. It has shown to be as useful with a variety of respiratory and non-respiratory specimens. Refinements to the cartridge called Xpert Ultra™ are likely to better the sensitivity further bringing it close to that of liquid culture [4]. Being a nested polymerase chain reaction (PCR), these tests also simultaneously provide information about Rifampicin resistance of the Mycobacterium tuberculosis (MTb) detected. Ease of testing, possibility of testing any type of respiratory specimens and rapidity of results are great potentials of this method. Despite the refinement, it however still falls short of the need of the profession. As no more than 40–50% of pediatric cases are culture-positive and a lesser or similar proportion are expected to be CBNAAT positive, thus, a negative CBNAAT does not rule out TB. Further, the test is far more expensive as compared to the smear (over 25–30 times) and requires electrical supplies and temperature control facilities. Under RNTCP, CBNAAT machines have been provided for free of cost testing all over country and upfront testing of all samples by this method is being advocated. Thus, rapid microbiological confirmation in pediatric samples is now a possibility.
Conventionally, all children suspected to have pulmonary TB would be subjected to a chest radiograph, Tuberculin skin test and possibly a smear test for sputum or gastric aspirate on the first visit despite a miserably low yield to effort for the smear test in children with primary disease. Now smear can, and should be replaced by more efficient CBNAAT even though it still can detect no more than 30–40% of childhood cases. Experience from the country shows that when the microbiological testing by CBNAAT is positioned like a sputum smear, right at the beginning for all those suspected to have TB, the yield of the test is dismally poor, under 10%. This is expected because the TB suggestive symptoms are common and lack specificity if not well characterised [5]. This raises an important question as to how this useful innovation can be properly utilised without wasting resources and overwhelming the laboratory system.
The evidence for a better positioning comes from some other studies. The yield improved several folds when respiratory secretions were tested only in cases where lesions were detected on chest radiology in a child with suggestive symptom. Delhi TB study group reported much higher yield of CBNAAT (22%) on their archived samples from clinico-radiologically diagnosed pediatric cases of pulmonary TB [6]. This is far more than the yield reported on upfront testing of the chest symptomatics referred to above. Several unpublished studies by the author have also found similar results. The pediatric TB experts thus agreed that CBNAAT could be used more efficiently if the respiratory specimens are tested in presumed cases with abnormal chest radiology.
CBNAAT has also shown fairly good to moderate sensitivity with extra-pulmonary specimens like pus, needle aspirates from the lymphnode, tissue biopsies, cerebrospinal fluid, etc. [7]. The yield is universally poor with pleural and ascitic fluid specimens. CBNAAT may be the initial test for aspirates from suspected TB lymphadenitis and the more challenging cytopathological diagnosis may be used in cases where the CBNAAT is negative for TB, particularly when the facilities for cytopathological testing are limited.
Universal Drug Sensitivity Testing (U-DST) and Testing for Drug Resistance
Under RNTCP, the current strategy is to have sensitivity guided therapy and thus universal drug sensitivity testing (U-DST) is recommended for all TB cases for early detection and appropriate treatment of the drug resistant cases (particularly the Rifampicin resistant cases). In the past, every new case was presumed drug sensitive and was initially given a standard regime of 4 first-line drug combination and the retreatment cases a 5 first-line drug regime. The scarcity of drug resistance testing facilities and the long time involved in testing, restricted the use of these tests for only those who were failing the retreatment regime or who interrupted treatment. The patients with drug resistance to Rifampicin and/ or Isoniazid (INH) were the commonest reasons for the failure of these regimes. The usage of standard therapy in such cases not only delayed their appropriate treatment but also contributed to further amplification and extended drug resistance.
The country has adopted robust technologies for rapid TB diagnosis and detection of resistance to Rifampicin and INH. This evolution now makes it possible to know the presence of resistance to these drugs upfront and plan an optimised therapy to improve cure rates and to prevent amplification of drug resistance, which was a risk with use of standard initial treatment regimens. The current technology (CBNAAT) employs a nested PCR which permits rapid simultaneous detection of Rifampicin resistance. This is very helpful as resistance to Rifampicin is a singular factor associated with the failure of first-line therapy and further amplification of resistance. So for all practical purposes at the current time, U-DST under RNTCP refers to upfront testing for rifampicin resistance.
Testing for resistance to INH, the other critical first-line drug, is possible with another approved molecular test belonging to the genre of line probe assay (LPA). LPA approved by WHO and used under RNTCP is GenoType MTBDR plus™ (Hain Lifescience GmbH, Nehren, Germany) and it uses PCR and reverse hybridisation assay to simultaneously detect TB and mutation associated with resistance to rifampicin as well as high and low level of INH resistance. In contrast to CBNAAT, this test is only suited for smear positive samples or on culture isolates and takes about 2 d of laboratory time.
More recently WHO has approved LPA for testing second-line drug resistance too (GenoType MTBDRsl™ from Hain Lifescience GmbH, Nehren, Germany) and it primarily tests for MTB diagnosis and class resistance to fluoroquinolones and second-line injectables. This test is recommended for all specimens showing Rifampicin resistance to select an appropriate drug regimen [8].
In summary, nested PCR in CBNAAT allows an upfront testing for both the mycobacterium and presence of Rifampicin resistance in all kinds of specimens at a much higher sensitivity even among smear negative cases, thus making it the preferred initial test for U-DST under the programme. Testing for INH resistance by LPA can only be done on smear positive specimens or on culture isolate. Thus, we are going to see major shift on positioning of sputum/ allied sample testing in the diagnostic algorithm for children. It is recommended that the CBNAAT testing of the respiratory specimens is done if only a presumed case demonstrates an abnormality on the chest skiagram, to get maximum yield to effort. For testing with CBNAAT, a single good quality specimen is recommended instead of the two specimens as required for the microscopy based methods.
Evolution of TB Drug Therapy under RNTCP
TB Drug Dosages
Studies commissioned by WHO and from within the country have revisited the pharmacokinetics (PKA) of all first-line TB drugs. This had led to an understanding that an upward revision of their dosages was needed for children. Pediatric patients show more rapid metabolism of Isoniazid as compared to adults and therefore require higher mg/kg body weight dose requirement [9,10,11,12]. More recent guidelines recommend an upward revision of the daily INH dose among children [13]. While the PKA data suggests much higher dosages for maximising area under curve above the minimum inhibitory concentration (MIC) for most first-line drugs, but there is lack of safety data when these drugs are used as a combination in higher doses, as many of the components have similar toxicity (e.g., INH, Rifampicin and Pyrazinamide are essential part of the current regimes and each of these can cause liver injury). Table 1 details the currently recommended doses as per the revised updated RNCP guidelines.
New Fixed Dose Combinations (FDCs)
FDCs incorporating the multi-drug therapy for TB are preferred due to safety and simplified treatment, no errors in missing one or more of the combination drugs, and thus a reduced risk of emergence of drug-resistant strains. From programmatic view point, it simplifies drug supply management, shipping and distribution. As the bioavailability of some of the component drugs, particularly Rifampicin can be affected when used in a combination formulation, FDC tablets of good quality and proven bioavailability of Rifampicin are required. Furthermore, with the upward revision of the first-line drug dosages, most existing FDCs do not have the appropriate dosing combination, In order to meet the needs of a wide range of weight bands, a low drug size combination pill has been developed with preferred ratio of H:R:Z as 1:1.5:3 for children. In order to decrease the pill burden and for ease of administration, the pediatric FDC formulations are developed as dispersible tablet (DT). Ethambutol being a hygroscopic drug is difficult to be formulated as a DT and hence is not part of the pediatric FDC DT [14].
Currently, two types of FDCs, which meet the WHO recommendations, are available under RNTCP. They are:
-
3 drugs pediatric FDC DT (H 50, R 75, Z150) (10:15:30) for children, (a separate non-DT Ethambutol 100mg tablet is added to complete the regime);
-
4 drugs FDC adult (H 75, R 150, Z 400, E 275).
The recommended therapy for the various weight bands using these FDCs per body weight is detailed in Table 2.
Adjunct Therapy- Pyridoxine
There has been a concern about need for pyridoxine in patients on ATT to prevent peripheral neuropathy. Isoniazid interferes competitively with pyridoxine metabolism by inhibiting the formation of the active form of the vitamin, and hence can result in peripheral neuropathy. It is difficult to recognise or diagnose peripheral neuropathy in young children but an unrecognised and untreated peripheral neuropathy can result in severe and prolonged morbidity. Given that our country has a high prevalence of malnutrition in children, particularly among those with active TB, hence it could be said that most of our patients are “at risk” of peripheral neuropathy with INH. This may be more so now due to currently recommended higher daily dosage of the drug. In the past, the pyridoxine supplementation was recommended only for high risk groups like human immunodeficiency virus (HIV), alcohol abuse, severe malnutrition, diabetes mellitus, renal or liver failure, and for MDR treatment. It was not recommended routinely for children. However, when intermittent regimen was being used under the RNTCP, the patients received pyridoxine on non-drug days to create a quasi-daily regime during the continuation phase so that they would not forget taking pills.
In light of the above, there has been a rethink and experts now recommend pyridoxine supplementation (10 mg per day) to all those receiving INH therapy. Low cost, safety and lack of any interference with INH action in the small prophylactic dose used, favour its use for its potential benefit.
Otherwise, no supplemental treatment in the form of multivitamin or multi-mineral is advised as there is no evidence that any of these improve the treatment outcome.
Addressing the Issue of Initial INH Resistance
Most countries, particularly the high TB burden and resource constrained nations, were guided by the erstwhile WHO guidance of using 2 major standard regimes for treating TB i.e., 4 drug regime for new cases and 5 drug retreatment regime for the relapse, failure and defaulters. There was little nationwide data on prevalence of drug resistance except that the resistance to Rifampicin or MDR was less than 5% among new cases and there was a wide variation of MDR TB among the retreatment cases with the RH (Rifampicin, Isoniazid) resistance being low among the relapses but it was higher among the failure and defaulters.
Gradually, programmatic data mounted to identify another problem related to drug resistance. Increasing evidence showed that the level of initial INH resistance was high among new cases and this could lead to amplification of resistance to Rifampicin as there was no effective companion drug for Rifampicin in the continuation phase. Thus, a need for adding a third companion drug (Ethambutol) to RH in the continuation phase of the primary regime for new cases was articulated in the Standards for TB care in India (2014) and later implemented under RNTCP [15].
WHO commissioned a systematic review on the outcomes of retreatment regime and it reported that the empiric use of this regimen in settings where resistance to Isoniazid and Rifampicin was unknown but likely to be high, led to unacceptably low rates of treatment success (median of 68%). Higher rates of poor outcomes and acquired drug resistance was reported among retreatment cases with confirmed isoniazid resistance when treated with category II regimen as compared to those who remained susceptible to isoniazid. There was also evidence to suggest that intermittent regimes, not fully supervised, may be associated with higher failures in the presence of INH resistance [16, 17].
This stepped way for shifting to a daily regime as was elucidated in the Standards of TB care in India (2014) and subsequently IAP-RNTCP guidelines for treating new childhood cases, in 2015. The programmatic changes were effected gradually by 2017 as it entailed nationwide training and procurement of new dose combinations for the daily regime. Ethambutol was added as a third drug to strengthen the continuation phase to improve cure rates and prevent amplification of resistance.
The initial belief which proposed a standard retreatment regimen was based on the reports that the resistance to the non-Rifampicin drugs in the initial therapy, can in most situations be overcome by a 8 mo, 5 drug retreatment regime, which had longer intensive phase (3 mo) and a 5 mo 3 drug continuation phase (RHE). Despite treating increasing number of new cases of TB with high cure rates under RNTCP strategy, evidence was building that the outcomes of retreatment (category II) was modest to poor and many of these cases had MDR TB [18,19,20]. Optimal therapy of these retreatment cases and prevention of further emergence of multidrug resistant TB was the new challenge.
With the addition of Ethambutol in the continuation phase, there was hardly any difference between the new and retreatment regime. This, coupled with moderate failures with category II, raised the demand for revisiting the therapy for retreatment cases. Simultaneously, the TB world was witnessing development and adoption of better robust rapid technologies to detect INH and Rifampicin resistance. As a result of better capacities to identify drug resistance, the standard retreatment regimen has been withdrawn and now all retreatment cases are put on optimised therapy based on their resistance pattern. In retreatment cases, where no resistance to H and R is detected, the initial drug regime is given again. While among those identified to have INH (mono or poly) resistance but no resistance to Rifampicin, a 6 mo monophasic regime constituted by Rifampicin, Levofloxacin, Pyrazinamide and Ethambutol is recommended [21]. This can be given for upto 12 mo in those who have central nervous system (CNS) and/or bone TB.
Management of Rifampicin Resistance in Children
With the availability of CBNAAT, patients having Rifampicin resistant (RR) disease can get identified early on. It is to be noted that R resistance is quite rare without H resistance. Majority of DST results with R resistance usually have H resistance, i.e., MDR TB. This has been substantiated in the recent National Drug Resistance Survey from our country [22]. Therefore, RNTCP has taken the programmatic decision that patients, who have any R resistance, should be managed as MDR TB case and this is in line with WHO global guidelines for Programmatic management of drug resistance TB (PMDT).
The drug therapy for MDR disease has recently undergone a lot of change and the recommended regimes are listed in the Table 3. This may require further change when the Drug Controller of India gives permission for the use of drugs like Bedaquiline among children. Globally, there is now a push towards having an all oral regime for treating MDR TB, which shall be possible once the newer drugs are approved for use in children.
Preventive Therapy
Children are more susceptible to TB infection, more likely to develop active TB disease soon after infection, and more likely to develop severe forms of disseminated TB. Children under 6 y of age, who are close contacts of a microbiologically confirmed pulmonary TB patient, need to be evaluated for active TB by a medical officer/ pediatrician as early as possible. After excluding active TB, INH preventive therapy is to be offered to such a child, irrespective of their BCG or nutritional status. The dose of INH for preventive therapy is 10 mg/kg body weight administered daily for a minimum period of six months.
INH preventive therapy is also recommended in the following situations:
-
For all HIV infected children who either had a known exposure to an infectious TB case or are Tuberculin skin test (TST) positive (> = 5 mm induration) but have no active TB disease.
-
All TST positive children who are receiving immunosuppressive therapy (e.g., Children with nephrotic syndrome, acute leukemia, etc.).
-
A child born to mother who was diagnosed to have TB in pregnancy should receive prophylaxis for 6 mo, provided congenital TB has been ruled out. BCG vaccination can be given at birth even if INH preventive therapy is planned.
To conclude, the management of TB among children has witnessed some important changes in the recent past. Optimised drug regimes shall now replace the standard treatment regimes. Universal upfront testing for Rifampicin resistance shall lead to early detection and proper management of the RR cases. This has largely been made possible with the advent of newer rapid detection molecular tests.
References
WHO. Roadmap towards ending TB in children and adolescents; 2018. Available at: https://www.who.int/tb/publications/2018/tb-childhoodroadmap/en/. Accessed 20th May 2019.
Management of Pediatric Tuberculosis under the Revised National Tuberculosis Control Program (RNTCP). A Joint Statement of the Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, and Experts from Indian Academy of Pediatrics. Indian Pediatr. 2004;41:901–5.
Bates M, O'Grady J, Maeurer M, et al. Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect Dis. 2013;13:36–42.
Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84.
Marais BJ, Obihara CC, Gie RP, et al. The prevalence of symptoms associated with pulmonary tuberculosis in randomly selected children from a high burden community. Arch Dis Child. 2005;90:1166–70.
Singh S, Singh A, Prajapati S, et al; Delhi Pediatric TB Study Group. Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiol. 2015;15:191.
Lawn SD, Zumla AI. Diagnosis of extrapulmonary tuberculosis using the Xpert® MTB/RIF assay. Exp Rev Anti-Infect Ther. 2012;10:631–5.
World Health Organization. The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs: policy guidance. 2016. Available at: http://www.who.int/iris/handle/10665/246131. Accessed 20th May 2019.
McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of south African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48:1547–53.
Thee S, Seddon JA, Donald PR, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother. 2011;55:5560–7.
Ramachandran G, Kumar AK, Swaminathan S. Pharmacokinetics of anti-tuberculosis drugs in children. Indian J Pediatr. 2011;78:435–42.
Mukherjee A, Velpandian T, Singla M, Kanhiya K, Kabra SK, Lodha R. Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in HIV-infected Indian children. Int J Tuberc Lung Dis. 2016;20:666–72.
Graham SM. Treatment of paediatric TB: revised WHO guidelines. Paediatr Respir Rev. 2011;12:22–6.
WHO, UNICEF Statement on the Use of Child-Friendly Fixed-Dose Combinations for the Treatment of TB in Children; 2017. Available at: https://www.who.int/tb/FDC_Factsheet.pdf. Accessed 20 May 2019
Standards for TB Care in India: Ministry of Health and Family Welfare, Government of India and WHO SEARO; 2014. Available at: https://tbcindia.gov.in/showfile.php?lid=3061. Accessed 20th May 2019.
Menzies D, Benedetti A, Paydar A, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000150.
Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:223–4.
Munje R, Deshmukh R, Tumane K. Multidrug-resistant TB among previously treated TB cases: a retrospective study in Nagpur, India. Indian J Tuberc. 2015;62:207–10.
Deepa D, Achanta S, Jaju J, et al. The impact of isoniazid resistance on the treatment outcomes of smear positive re-treatment tuberculosis patients in the state of Andhra Pradesh, India. PLoS One. 2013;8:e76189.
Srinath S, Sharath B, Santosha K, et al. Tuberculosis 'retreatment others': profile and treatment outcomes in the state of Andhra Pradesh, India. Int J Tuberc Lung Dis. 2011;15:105–9.
WHO Treatment Guidelines for Isoniazid Resistant Tuberculosis: Supplement to the WHO Treatment Guidelines for Drug-Resistant Tuberculosis. Available at: https://www.who.int/tb/publications/2018/WHO_guidelines_isoniazid_resistant_TB/en/. Accessed 20 May 2019
Report of First National Anti-TB Drug Resistance Survey of India (2014–16). Ministry of Health and Family Welfare, Government of India. Available at: https://tbcindia.gov.in/showfile.php?lid=3315. Accessed 20th May 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, V. Pediatric TB Management under RNTCP: What and Why?. Indian J Pediatr 86, 707–713 (2019). https://doi.org/10.1007/s12098-019-03001-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-03001-7