Abstract
The timing and role of chemotherapy in the management of Wilms’ tumor has long been the matter of debate, with different groups showing equally comparable and encouraging results. Over the last decade, however, both the ideol-ogies seem to be converging and the attempt has been to identify groups benefitting with pre-operative chemotherapy, as well as those, where upfront resection should be attempted. In this article authors intend to discuss pros and cons of both the strategies and their applicability in a resource poor setting in developing countries like India.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wilms’ tumor (WT) is the most common primary renal malignancy in children. It is one of the commonest childhood cancers where persistently innovating multimodal strategies have led to conversion of an almost uniformly fatal disease to one with excellent survival. Presently, the survival of localized disease has been around 90% and in metastatic disease it has increased up to 70% [1]. The paradigm shift in the understanding of management of the disease has largely been driven by multiple trials done by two major groups i.e., National Wilms Tumor Study/Children’s Oncology Group (NWTS/COG) and International Society of Pediatric Oncology (SIOP), endorsing the North American and European school of thoughts, respectively. The other smaller groups like United Kingdom Children’s Cancer Study Group (UKCCSG) have also contributed immensely to the understanding of the disease and its management.
History
The initial work in 1950s by Dr. RE Gross, a very influential Chief Surgeon at Boston Children’s hospital, in North America set the tone of wide recognition of upfront surgery approach [2]. Next generation pediatric surgeons imbibed his beliefs of high morbidities associated with pre-resection radiotherapy, and this led to upfront resection being backbone of all further studies done by NWTS. During the same time, clinicians in Europe developed a strategy of pre-operative radiotherapy. This school of thought developed in Paris initially, where experience came from managing many patients coming from North America who presented with large tumors in poor general conditions [3]. Radiotherapy shrank the tumors and gave time to improve general well-being of children. The investigators from SIOP adopted this school of thought, where further refinements led to chemotherapy replacing radiotherapy in upfront setting.
NWTS Approach: Resection First
The primary advantage of this strategy is that it allows accurate assessment of histology and tumor stage. The other information achieved is an unadulterated tissue for pathological and molecular assessment. Besides, a number of patients of benign or non-WT histology can be saved from inadvertent therapy. In SIOP- 9 the incidence of non-WT histology was 5% (28/511) including 1.6% benign cases [4]. The primary concerns with this strategy have been increased tumor rupture rates and peri-operative surgical complications. NWTS was involved in five clinical trials i.e., NWTS 1 to NWTS 5. In 2001, it merged with several other pediatric groups to form Children’s Oncology Group (COG). Further trials are being run by COG.
NWTS-1 (1969–75) concluded that post-operative ab-dominal radiotherapy is not necessary for children less than 2 y of age with completely resected tumors limited to the kidney. In addition, the combination of vincristine and dactinomycin was shown to be more effective for the treatment of children with tumors that extended beyond the kidney than either drug alone [5]. NWTS-2 (1975–79) demonstrated that 6 mo of two drugs i.e., actinomycin D (ACD) and vincristine (VCR), combination chemotherapy was effective treatment of children with tumors limited to the kidney and completely resected [6]. The separation of Wilms’ tumor into distinct histopathologic categories based on prognosis was used to stratify patients in NWTS-3 (1979–86). This study also began to define the low dose of ionizing radiation to be used, when necessary, and showed that the addition of cyclophosphamide did not improve survival over that generated with three-drug therapy [7]. NWTS-4 (1986–95) examined the utility of dose intensive scheduling to cut down on the duration of therapy [8]. NWTS-5 (1995–2002), evaluated the prognostic value of certain biologic markers in Wilms’ tumor, and showed that Loss of heterozygosity (LOH) on chromosomes 1p and 16q in stage I and II favorable histology Wilms’ tumor was associated with a poorer prognosis [9].
COG / NWTS is running multiple clinical trials and the final results are pending. AREN0532 study is re-evaluating the futility of chemotherapy and surgery only approach for stage I favorable histology (FH), age < 2 y, tumor <550 g.
AREN0533 study has evaluated treatment of newly diagnosed higher risk favorable histology Wilms’ tumors. Chemotherapy intensification is done in presence of adverse molecular markers (LOH 1p and 16q). Radiotherapy and chemotherapy intensification is done as per resolution of pulmonary lesions and or presence of adverse molecular features.
AREN0534 study has evaluated the role of pre-operative chemotherapy in the treatment of patients with bilateral, multi-centric, or bilaterally predisposed unilateral Wilms’ tumor.
SIOP: Pre-operative Chemotherapy
The SIOP strategy of giving pre-operative chemotherapy is based on the premise that pre-operative therapy reduces the risk of tumor rupture during surgery, thereby decreasing the likelihood of local and distant recurrence. Besides, as the staging is done after surgery which is done after pre-operative chemotherapy, the down staging of tumors lead to avoidance of intensified therapy in a large number of patients. Since 1971, multiple SIOP studies have been conducted to determine optimal pre-operative chemotherapy regimen and duration in pre-operative setting. SIOP 1 (1971–74) demonstrated that pre-operative radiotherapy (RT) in comparison with upfront resection reduces incidence of rupture (32% to 4%) and downstage the tumor post surgery (stage 1–14% vs. 31%). Prolonged courses of Actinomycin D post surgery did not increase disease free survival (DFS) [10]. SIOP 2 (1974–76) showed that 9 mo of post-operative two-drug adjuvant therapy (VCR + ACD) is equal to 15 mo. Hence, shorter duration is adequate. Even in small tumors, pre-operative radiotherapy led to decrease in incidence of rupture rates [11]. SIOP-5 (1977–79) demonstrated that two-drug neo-adjuvant chemotherapy (VCR + ACD) for 4 wk is comparable to pre-operative radiotherapy and one course of ACD [12]. Thus chemotherapy was found to be preferable in view of long term toxicities associated with radiotherapy. SIOP-6 (1980–87) confirmed that in stage-I, 17 wk of two-drug therapy is equivalent to 38 wk of therapy. In stage II N+ and stage III, addition of Doxorubicin led to better DFS. In stage II N0, there was increased incidence of local relapses in non-irradiated arm; hence this arm was closed early [13, 14]. SIOP-9 (1987–91) confirmed that 4 wk of two-drug chemotherapy is equivalent to 8 wk in localized tumors. Results were equivalent in relation to down staging, rupture rates, DFS and overall survival (OS) [14]. SIOP (1993–2001) met its objective of proving that shorter duration (4 wk) of post-operative chemotherapy is comparable to longer schedule (18 wk) for patients with stage I intermediate-risk and anaplastic Wilms’ tumor [15].
Major Issues
Tumor Spillage
The major difference in the two groups of clinicians has been the issue of tumor spillage during upfront surgery. Increased incidence of tumor spillage of ruptures lead to increased abdominal relapses [16]. Early studies revealed incidence of tumor spillage in excess of 30%. Pioneer studies by European group SIOP 1 (1971–74) revealed a tumor rupture rate of 4% in pre-operative radiotherapy group vs. 32% in upfront surgery group. In SIOP-5 the incidence was 6%; the study showed only gross tumor spillage. On the other hand, NWTS–4 study had an incidence of 20%, which included local tumor spillage [7].
Though all cases of tumor rupture might not lead to relapses, as Kalapurakal et al. (unpublished data) showed the implantation rate for spilled cells to less than 20% [17]. To add to the scenario, NWTS-4, showed such group of patients had increased incidence of relapse but similar overall survival [18]. However, despite comparable survival, it is better to avoid spill. Spill will lead to longer and costlier chemotherapy regimen with the addition of another drug (Doxorubicin) and radiotherapy.
Upfront Biopsy
Both the groups do not endorse upfront tissue diagnosis before starting either sort of treatment. The dangers of tumor rupture and thus upstaging of the tumor has long been the matter of concern. The issue is more associated with SIOP strategy, where chemotherapy is started on the basis of characteristics image findings. UKCCSG study showed that the incidence of non-WT histology in children with renal masses has been around 15% on pre-chemotherapy biopsy specimen. Only in 4% cases, it was not diagnostic [19]. The implications of attempts at histological diagnosis looks more convincing for renal tumors with atypical presentation like younger (< 6 mo), older age (>3.5 y), significant retroperitoneal adenopathy, intratumoral calcification, absence of any renal parenchyma on imaging or uncommon sites of metastases (brain or bone). On the other hand, the UKCCSG has long been advocating core needle biopsy of such tumors upfront before instituting chemotherapy and they have shown that such patients do not have increased tumor rupture, needle tract seeding or tendency to relapse. Though, further management by this group is influenced by SIOP strategy.
Convergence of Approach: SIOP and NWTS
There are a few situations when upfront surgery is the only recommended option. Studies have shown that the incidence of Wilms’ tumor in infants with renal mass is significantly lower and imaging is not helpful in this situation. van den et al., in a retrospective analysis showed that among 750 infants less than 7 mo, 34.5% had histology other than WT [20]. Congenital mesoblastic nephroma was found to be present in 18% of cases and 8% cases were of malignant rhabdoid tumor.
The management of stage I favorable histology tumors in children aged less than 2 y has been redefined by COG. In NWTS-5 such patients when treated with surgery alone, were found to have event free survival (EFS) of 86.5% and this arm was closed in view of it being lower than predefined EFS of 90%. However, the follow-up of these patients revealed that the 5-y overall survival was equal in both the arms. All the patients who had relapsed could be salvaged. This approach was again reinstated in further COG protocols. The recently completed COG AREN0532 study is again analyzing this approach.
Bilateral tumors comprise 6% of the total cases and they pose a complicated challenge during the management. The intent has been to decrease the size and extent of tumors so that maximum healthy renal parenchyma could be preserved during resection (Nephron sparing surgery – NSS). Nephron sparing surgery following neo-adjuvant chemotherapy has now been established as a reasonable approach for all cases of bilateral WT. Recent trial by COG, AREN0534 has incorporated 3-drug chemotherapy for 6–12 wk before re-assessment for surgery in such cases. Paulino et al. showed that 3 drug regimen lowers relapse rate in comparison to 2 drug regimen [21]. However, SIOP- 2001 trial still incorporates 2 drugs upfront. The utility of a third drug in this setting is still unresolved. In cases of Wilms’ tumor in a solitary kidney, or unilateral tumor with a risk for metachronous tumor on other side (in genetic syndromes associated with WT1 and WT2 genes), the approach of NSS following neo-adjuvant chemotherapy is accepted way of treatment.
Despite an aggressive upfront surgical approach by North American groups, the recent COG studies have incorporated surgeons’ judgment for eligibility for resection. Tumors with inferior vena cava (IVC) thrombus above level of hepatic veins or large tumors with involvement of contiguous organs, often lead to reluctance for upfront surgery. The aim has been to avoid spill, residual disease and surgical complications. Twenty-three percent of patients in AREN0532 underwent delayed resection and around 40% patients in AREN0533 were addressed similarly.
Indian Perspective
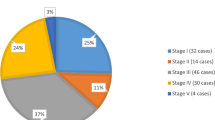
There is paucity of data from the developing countries. However, the available data does suggest that majority of tumors are large and in advanced stages at presentation [22, 23]. Majority of patients have poor nutritional status and general condition. The scenario in India is no different. A study from authors’ centre showed that around 75% of cases present in advanced stages i.e., stages III, IV and V [24]. Delayed presentation to a specialist care centre is a common scenario in this part of the world. As there are no uniform guidelines in India, the treatment has been more dependent on the physicians’/centers’ choice. Often patients presenting to the surgeons are operated first while those presenting to the oncologists are put on neo-adjuvant chemotherapy. High rates of abandonement in this part of the world has also contributed to the overall poorer outcome in comparison to the western world [25].
At authors’ centre, over the years, they have developed their own protocol, which consists of upfront tissue diagnosis in all patients, who are to be started on neo-adjuvant chemotherapy, in the form of fine needle aspiration cytology (FNAC). A study on FNAC, by Iyer [26], from authors’ centre showed 110 patients to be cases of WT among a total of 119 children with suspected renal mass (92.4%). Eight cases were of clear cell sarcoma. At authors’ center, in addition to the criteria for neo-adjuvant chemotherapy as defined by NWTS, patients with large tumors, tumors that are fixed on CECT scan evaluation, any IVC thrombus and poor general condition are started on neo-adjuvant chemotherapy strategy. Agarwala et al. had reported, in 2009, that using this selection criteria, 45% of patients had received neo-adjuvant chemotherapy. The overall survival was 88.6% in upfront surgery group vs. 77.8% in pre-resection chemotherapy group [24]. The difference in outcome could be because of the inherent aggressive nature of tumors making it un-resectable upfront. As a whole, the overall survival was 100% for stage 1, 85.7% for stage 2, 85.7% for stage 3 and 63.2% for stage 4. In an another study, the same authors showed incidence of inferior vena-caval thrombus to be around 9% and a 5-y overall survival of 50% in this subset in a neo-adjuvant setting [27]. The authors follow their institutional chemotherapy protocol comprising of neo-adjuvant chemotherapy for selected patients. Three-drug neo-adjuvant therapy (VCR + ACD + DOX) is followed by surgery at 6 wk and further post-operative adjuvant chemotherapy ± radiotherapy as per staging. These patients who receive neo-adjuvant chemotherapy are not down staged but are treated as minimum stage III or stage IV (if metastases are present). This is as recommended by the NWTS for patients receiving neo-adjuvant chemotherapy. Smaller studies from other centers from the country, suggest that different centers have adopted their own strategies [28, 29]. However, there is lack of large multicentric data and the patient population is heterogenous.
The applicability of pre-resection chemotherapy seems to be more relevant in the Indian scenario. Lesser availability of surgical expertise and longer waiting dates at radiotherapy centers in our country might favor this approach of down staging the tumors following neo-adjuvant chemotherapy (SIOP approach). The authors experience has shown that a meticulate selection of patients for upfront surgery and neo-adjuvant chemotherapy in rest of the cases can result in outcome comparable to the developed world. However, they have still not resolved if these patients should be down staged (as recommended by SIOP) or not. The SIOP guidelines for Pediatric Oncology in Developing Countries (PODC) have made several minimum recommendations [30]. These include centers with basic laboratory and radiology services, with provisions for essential chemotherapy drugs and facilities for safe administration, trained surgeon and availability of supportive care as well as social support. Most oncology centers in India more than fulfill these criteria, except possibly financial and social support. Recommendations pertinent to our scenario include administration of pre-operative chemotherapy in large tumors, starting with a lower dosage of drugs (2/3rd) in severely acutely malnourished children and reduction of Doxorubicin dose to 30 mg/m2 in case of neutropenia. Indian Pediatric Oncology Group (InPOG) was formed to evolve nation wide studies for various tumors [31]. The Renal tumor study group of InPOG has recently proposed a study that recommends the SIOP approach of neo-adjuvant chemotherapy for all WT patients in India. Recently, Indian Council of Medical Research (ICMR) has also formulated guidelines for the treatment of WT in India [32]. This has also favored neo-adjuvant chemotherapy for majority of WT patients.
Discussion
The debate between North American and European strategies has continued for last 25 y. The survival data has been comparable in both the approaches. UKCCSG group actually compared the strategies in a trial setting and confirmed their equivalent efficacies. The most important prognostic factors in the management of Wilms’ tumor are tumor stage and histology. The North American group has been the pioneer of the upfront strategy and the argument lies in the notion that upfront surgery demonstrates the true stage and histology of tumors. It also solves the problem of upfront histological diagnosis in renal masses, thus avoiding unnecessary chemotherapy in non-WT tumors. However, improved imaging techniques have led to decreased incidence of false positive diagnosis as seen in SIOP 9 study (5%). The UKCCSG strategy of upfront needle biopsy is also meaningful in areas where expertise is available.
The problem with increased tumor spill and thus upstaging of tumors in NWTS strategy has led support to European strategy endorsed by SIOP. Over the years many studies by SIOP have demonstrated decreased rupture rates in neo-adjuvant setting. However, it is important to note that increased rupture rates only result in increase in relapses but the overall survival remains same if appropriately treated. The observations at authors’ institution have not shown significant decrease in the tumor spillage rate after neo-adjuvant chemotherapy (26% vs. 15%; p = 0.16) [33]. This is probably because the patients present late with massive tumors, which reduce in size significantly, but probably not enough to significantly decrease the operative tumor spillage. Neo-adjuvant chemotherapy also makes these large tumors necrotic and very adherent to surrounding structures, and therefore more prone to rupture during operative manipulations. Further the 5-y OS (84% vs. 82%; p = 0.97) and relapse free survival (RFS) (79% vs. 76%; p = 0.72) for those with spill and without spill was also not significantly different [33].
Pre-operative chemotherapy also leads to decrease in surgery related complications. In SIOP-9, the rate of complication other than rupture was 8% while in NWTS-3 study, the incidence of surgical complications ignoring intra-operative tumor rupture was 19.8% in patients who underwent primary nephrectomy [34,35,36].
The other support for SIOP comes from the advantages achieved from down staging of the tumors and thus, de-intensification of the treatment. The adjuvant chemotherapy management is dependent on the histology of surgical specimen. SIOP has developed a separate classification comprising of good, intermediate and high risk groups necessitating intensified chemotherapy accordingly. This pathological classification may be difficult to perform in most centers in India as it requires intensive sectioning of the entire specimen.
However, the SIOP strategy is not devoid of concerns. Clonal evolution of such tumors has been shown in past and post chemo histology might not represent the true nature of the tumor [37]. Besides in SIOP-6 there were increased abdominal relapses in stage II node negative group, indicating that those were true stage III. To decrease the incidence SIOP has intensified the therapy in this by adding anthracyclins. However, this means that there is a possibility of a good number of real stage II patients getting unnecessary third drug, though some are saved from radiotherapy.
Despite both groups advocating a diagonally opposite approach, recent studies over last one and a half decade have resulted in borrowing of ideas from each other. The main goal of all the recent studies has been to de-intensify therapy in low risk group, identify appropriate well-defined risk factors and increasing survival in poor risk group by intensification of the therapy. COG has incorporated surgeon’s preferences in their recent studies. In patients presenting with lung metastasis, stage IV, NWTS group has modified its strategy of lung irradiation in 100% cases and incorporated the SIOP idea of chemotherapy induced response directed radiation. COG AREN0533 study is evaluating this strategy.
Conclusions
The debate between pre-operative chemotherapy vs. upfront resection should be analyzed in perspective. In developing countries like ours, where clinicians not only have to fight against tumors but also against delayed presentations, poor nutritional status and paucity of experienced centers, the importance of neo-adjuvant chemotherapy increases many folds. It is therefore, prudent to recommend neo-adjuvant chemotherapy for all patients of WT in India. However, high volume centers, that have developed expertise over the years, can develop the best strategy suited to their population.
References
Spreafico F, Bellani FF. Wilms’ tumor: past, present and (possibly) future. Expert Rev Anticancer Ther. 2006;6:249–58.
Gross RE. The surgery of infancy and childhood. Philadelphia: W.B. Saunders Co; 1953. p. 1000.
Schweisguth O, Bamberger J. Nephroblastoma in children (in French). Ann Chir Infant. 1963;4:335–54.
Tournade MF, Com-Nougué C, De Kraker J, et al. Optimal duration of pre-operative therapy in unilateral and non-metastatic Wilms tumour in children older than 6 months: results of the ninth International Society of Paediatric Oncology Wilms Tumour Trial and Study. J Clin Oncol. 2001;19:488–500.
D’Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms’ tumor: results of the National Wilms’ Tumor Study. Cancer. 1976;38:633–46.
D’Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms’ tumor: results of the second National Wilms’ Tumor Study. Cancer. 1981;47:2302–11.
D’Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms’ tumor. Results of the third National Wilms’ Tumor Study. Cancer. 1989;64:349–60.
Green DM, Breslow N, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: a report from the National Wilms’ tumor study group. J Clin Oncol. 1998;16:237–45.
Grundy PE, Breslow NE, Li S, et al; National Wilms Tumor Study Group. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–21.
Lemerle J, Voute PA, Tournade MF, et al. Preoperative versus postoperative radiotherapy, single versus multiple courses of Actinomycin D in the treatment of Wilms’ tumor. Preliminary results of a controlled clinical trial conducted by the International Society of Paediatric Oncology (SIOP). Cancer. 1976;38:647–54.
De Kraker J, Graf N, Pritchard-Jones K, et al. Nephroblastoma clinical trial and study SIOP 2001, Protocol. SIOP RTSG. 2001.
Lemerle J, Voûte PA, Tournade MF, et al. Effectiveness of preoperative chemotherapy in Wilms’ tumor: results of an International Society of Paediatric Oncology (SIOP) clinical trial. J Clin Oncol. 1983;1:604–9.
Tournade MF, Com-Nougué C, Voûte PA, et al. Results of the Sixth International Society of Paediatric Oncology Wilms tumor trial and study: a risk adapted therapeutic approach in Wilms tumor. J Clin Oncol. 1993;11:1012–23.
Jereb B, Burgers MV, Tournade MF, et al. Radiotherapy in the SIOP (International Society of Paediatric Oncology) nephroblastoma studies: a review. Med Pediatr Oncol. 1994;22:221–7.
de Kraker J, Graf N, van Tinteren H, et al. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet. 2004;364:1229–35.
Burgers JM, Tournade MF, Bey P, et al. Abdominal recurrences in Wilms’ tumours: a report from the SIOP Wilms’ tumour trials and studies. Radiother Oncol. 1986;5:175–82.
D’Angio GJ. Pre- or postoperative therapy for Wilms’ tumor? J Clin Oncol. 2008;26:4055–7.
D'Angio GJ. Pre- or post-operative treatment for Wilms' tumor? Who, what, when, where, how, why-and which. Med Pediatr Oncol. 2003;41:545–9.
Vujanić GM, Kelsey A, Mitchell C, Shannon RS, Gornall P. The role of biopsy in the diagnosis of renal tumors of childhood: results of the UKCCSG Wilms tumor study 3. Med Pediatr Oncol. 2003;40:18–22.
van den Heuvel-Eibrink MM, Grundy P, Graf N, et al. Characteristics and survival of 750 children diagnosed with a renal tumor in the first seven months of life: a collaborative study by the SIOP/GPOH/SFOP, NWTSG, and UKCCSG Wilms tumor study groups. Pediatr Blood Cancer. 2008;50:1130–4.
Paulino AC, Wilimas J, Marina N, et al. Local control in synchronous bilateral Wilms tumor. Int J Radiat Oncol Biol Phys. 1996;36:541–8.
Ekenze SO, Agugua-Obianyo NEN, Odetunde OA. The challenge of nephroblastoma in a developing country. Ann Oncol. 2006;17:1598–600.
Abuidris DO, Elimam ME, Nugud FM, Elgaili EM, Ahmed ME, Arora RS. Wilms tumour in Sudan. Pediatr Blood Cancer. 2008;50:1135–7.
Agarwala S, Bakhshi S, Srinivas M, et al. 41st Annual Conference of International Society of Paediatric Oncology SIOP 2009, Sao Paulo, Brazil, October 5–9, 2009. Wilms tumor: management strategies and outcomes of AIIMS-WRT-99 Trial. Pediatr Blood Cancer. 2009;53:712.
Koodiyedath B, Kulkarni KP, Arora RS. Outcomes of Wilms tumour in India: findings from a systematic analysis: PH039. Pediatr Blood Cancer. 2012;59:1061.
Iyer VK. Role of fine needle aspiration cytology in the management of pediatric renal tumors. J Indian Assoc Pediatr Surg. 2007;12:116–9.
Moorthy G, Agarwala S, Bakhshi S, et al. Long term outcome of children with wilms tumor having intravascular thrombus. J Indian Assoc Pediatr Surg. 2009;14:152.
Trehan A, Chowdhary SK, Marwaha RK. Wilms tumor: five-year tumor-free survival on a modified SIOP protocol from an Indian university hospital. J Pediatr Hematol Oncol. 2012;34:57–62.
Guruprasad B, Rohan B, Kavitha S, Madhumathi DS, Lokanath D, Appaji L. Wilms’ tumor: single centre retrospective study from South India. Indian J Surg Oncol. 2013;4:301–4.
Israels T, Renner L, Hendricks M, Hesseling P, Howard S, Molyneux E. SIOP PODC: recommendations for supportive care of children with cancer in a low-income setting. Pediatr Blood Cancer. 2013;60:899–904.
Arora RS, Bakhshi S. Indian pediatric oncology group (InPOG) – collaborative research in India comes of age. Pediatr Hematol Oncol J. 2016;1:13–7.
Prasad M, Vora T, Agarwala S, et al. Management of Wilms tumor: ICMR consensus document. Indian J Pediatr. 2017; doi:10.1007/s12098-017-2305-5.
Agarwala S, Bajpai M, Bhatnagar V, et al. Effect of tumor spill on recurrence rate and outcome in patients with wilms tumor: results of AIIMS-WT 99 study. J Indian Assoc Pediatr Surg. 2009;14:151.
Godzinski J, Tournade MF, de Kraker J, et al. Rarity of surgical complications after post chemotherapy nephrectomy for nephroblastoma. Experience of the International Society of Paediatric Oncology — trial and study “SIOP-9”. International Society of Paediatric Oncology Nephroblastoma Trial and Study Committee. Eur J Pediatr Surg. 1998;8:83–6.
Schamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg. 1999;229:292–7.
Ritchey ML, Kelalis PP, Breslow N, et al. Surgical complications after nephrectomy for Wilms’ tumor. Surg Gynecol Obstet. 1992;175:507–14.
Hill DA, Shear TD, Liu T, Billups CA, Singh PK, Dome JS. Clinical and biologic significance of nuclear unrest in Wilms tumor. Cancer. 2003;97:2318–26.
Author information
Authors and Affiliations
Contributions
AJ: Review of literature and drafting the manuscript; SA: Review of literature, prepared the final draft of the manuscript and will act as guarantor for the paper; SB: Review of the paper and medical oncology inputs.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Kumar, A., Bakhshi, S. & Agarwala, S. Is Pre-operative Chemotherapy Desirable in all Patients of Wilms’ Tumor?. Indian J Pediatr 84, 709–714 (2017). https://doi.org/10.1007/s12098-017-2410-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2410-5