Abstract
Space occupying liver lesions usually present with abdominal pain or abnormal physical findings, such as a palpable abdominal mass or distention. Liver lesions identified in children include benign and malignant neoplasms, inflammatory masses, cysts and metastatic lesions. Two-thirds of liver lesions in children are malignant. Hepatoblastoma accounts for two-thirds of malignant liver tumors in children. Benign lesions of the liver in children include vascular lesions, hamartomas, adenomas, and focal nodular hyperplasia. Although benign and malignant liver masses share some clinical manifestations, however treatment and prognosis differ. Evaluation involves physical examination, imaging evaluation and laboratory investigations such as serological markers [alpha-fetoprotein (AFP)] for malignant liver lesions. Ultrasound is the initial imaging modality of choice because it can detect, characterize, and provide the extent of liver lesions. However, CT or MRI are often subsequently performed for further characterization, assessment of precise extent, and detection of associated metastatic disease in cases of malignant hepatic neoplasm. Serological markers (such as alpha fetoprotein) can be useful in narrowing the differential diagnosis when they are markedly elevated but a substantial number of patients unfortunately do not have high levels of these markers at the time of presentation or cautious interpretation is warranted as AFP level is frequently elevated in infants up to 6 mo of age and may be slightly elevated with benign tumors and with hepatic insult or regeneration. Therefore, a tissue diagnosis is often required to guide subsequent management. The histology and anatomy of a pediatric liver tumor guides the treatment and prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Space occupying lesions in the liver present a relatively common clinical dilemma, particularly with the increasing use of various imaging modalities in the initial assessment of children presenting clinically with deranged liver function tests or non specific abdominal symptoms [1, 2]. The management of pediatric liver lesions may be challenging and it may require a complete work-up because of symptoms or concern about malignancy. Initial evaluation should be focused on patient history, gestational history and age, weight and findings on physical exam. Diagnostic imaging modalities may facilitate the identification of benign and malignant liver tumors, however biopsy or resection for histological diagnosis sometimes becomes necessary [3]. Standard histologic examination usually is complemented by immunohistochemical analysis of protein biomarkers. Some of the infantile hepatic neoplasms are highly vascularized and surgical interventions are at high risk of bleeding. Certain tumor markers may be helpful in the initial work-up and evaluation of response to therapy. Alpha-fetoprotein (AFP) level may be elevated in children with malignant lesions such as hepatoblastoma and hepatocellular carcinoma, but cautious interpretation is warranted as AFP level is frequently elevated in infants up to 6 mo of age and may be slightly elevated with benign tumors and with hepatic insult or regeneration [3, 4]. Therapy must be tailored according to the nature of the lesion. Observation is recommended for asymptomatic hepatic hemangioma, whereas complete surgical resection is the mainstay of treatment in hepatoblastoma [4]. Unresectable metastatic masses require oncologic consultation and therapy. The efficient characterization and management of liver lesions therefore requires a multidisciplinary collaboration between the hepatologist, radiologist, pathologist, hepato-biliary or transplant surgeon, and pediatric oncologist.
Initial Clinical Evaluation
A careful review of the personal history and physical examination findings often helps in narrowing the differential diagnoses of liver lesions. A history of constitutional symptoms such as fever may be useful in the diagnosis of hepatic abscesses; although fever can also be associated with malignancy.
The physical examination may show features of chronic liver disease such as spider angiomas, periumbilical caput medusa indicative of portal hypertension, hepatomegaly, or splenomegaly.
Presence of skin hemangiomas raises the possibility of liver lesions likely to be vascular lesions. The family history is also of value in the initial clinical evaluation. A family history of young-onset diabetes mellitus, for example, may predispose to hepatic adenomatosis.
Most children with a primary liver tumor present with abdominal distention and a palpable mass in the upper abdomen often without other signs of severe disease. Anemia is often present [1]. Only in advanced stage of disease, overall status deteriorates and the children develop abdominal pain, weight loss, nausea, vomiting, and ascites. Jaundice, signs of hepatic insufficiency or an incidental rupture of the tumor with intraabdominal bleeding are very rarely observed. Many tumors may have reached a considerable size before they are noticed and treatment initiated [5]. However, some specific symptoms are associated with the different tumors, such as fever and thrombocytosis with hepatoblastoma and precocious puberty secondary to human chorionic gonadotropin or rarely testosterone production in hepatoblastoma or germ cell tumors. High output cardiac insufficiency due to arterio venous shunting in the lesion and platelet sequestration and consumptive coagulopathy (Kasabach-Meritt syndrome) can be encountered in young infants with a hemangioma or hemangioendothelioma of the liver, who often show hemangiomas of the skin and organs [5].
Differential diagnosis of liver lesions in neonates seems to be particularly difficult because diagnostic criteria and tumor markers do not apply and imaging is nonspecific. Rarely, hepatic choriocarcinoma can occur in neonates, clinically resembling infantile hemangioendothelioma but secreting beta HCG. Malignant Rhabdoid tumors can become a differential diagnostic problem to hepatoblastoma in infants and young children and benign teratomas have to be differentiated from mesenchymal hamartomas in the same age group.
Children with background genetic or metabolic disorders or infectious disease can develop liver lesions [4]. Most important are hepatoblastoma in children with a Beckwith-Wiedmann syndrome or other hemi-hypertrophy syndromes, familial polyposis coli and children with very low birth weight. Hepatocellular carcinoma can occur in endemic areas with perinatal hepatitis B or C infection or chronic liver diseases.
Laboratory Investigations
The history and physical examination are complemented by laboratory tests that may show active hepatitis, a low platelet count caused by chronic liver disease with cirrhosis, portal hypertension and hypersplenism, or hyperbilirubinemia.
The most important tumor marker is alpha-fetoprotein (AFP), which is highly elevated in 80 to 90 % of all hepatoblastomas and moderately elevated in 50 % of hepatocellular carcinoma (HCC) patients. It can also be highly elevated in malignant teratoma and yolk sac tumors of liver. AFP normal levels can exceed 500,000 ng/ml in neonates and 300 ng/ml in 3-y-old children. Neonatal hepatoblastomas do not produce enough AFP to produce serum levels markedly above the normal range. A slightly elevated level of AFP is found in other tumors, after damage or during regeneration of liver parenchyma [4]. Other markers elevated are listed in Table 1.
Virological titers should be investigated for hepatitis B, and C (HCC) [6], HIV-1 (Fibrosarcoma), cytomegaly and EBV (Lymphoma).
Role of Imaging
The initial modality of choice in imaging space occupying lesion in the liver is ultrasound (US). The lesion can be localized to the liver, characterized as solid or cystic on grey scale and vascularity within and around the lesion can be gauged with color/power Doppler [3, 7]. Doppler also helps to determine vascular invasion or thrombosis. It can be performed without anesthesia and repeated often for follow up, as it does not involve ionizing radiation. US is the recommended technique for follow-up [8]. Contrast enhanced ultrasound (CEUS) is increasingly promising in demonstrating the benign nature of focal liver lesions that are indeterminate on grey-scale sonography in children, potentially reducing the use of CT [1, 9]. US-guided liver biopsy in children is a procedure with a low rate of major complications and a high rate of minor bleeding that does not require intervention [9].
The role of advanced imaging tests such as CEUS and Promovist enhanced magnetic resonance imaging, which allow for non-invasive assessment of liver tumors, is of utmost importance in pediatric patients, especially when repeated imaging tests are needed and radiation exposure should be avoided.
CT and MRI help in identifying the lesion as either solitary or multifocal and best delineate the size and margins of the liver lesion [1]. MRI with contrast enhancement may provide the best identification of flow characteristics and surrounding vascular structures without ionizing radiation risk of CT [8].
Accurate characterization of liver masses by cross-sectional imaging is particularly dependent on an understanding of the unique phasic vascular perfusion of the liver and the characteristic behaviors of different lesions during multi- phasic contrast imaging. Cross-sectional imaging with CT or MRI is enhanced by the use of intravenous contrast agents and dynamic multiphasic examination techniques. The liver has 3 distinct phases after intravascular contrast agent is injected via a peripheral vein. The arterial phase occurs 10 to 15 s after peripheral contrast injection and is caused by the direct infusion of arterial blood with a high concentration of contrast from the heart through the hepatic artery into the liver. Next, the portal venous phase occurs 60 to 75 s after contrast injection as blood from the gastrointestinal tract is collected in the portal vein for processing in the liver. Finally, in the venous phase, blood from the liver is collected into the hepatic veins, which converge to the inferior vena cava for return to the right atrium. The intravascular contrast leaks through the liver sinusoids into the extracellular space and about 3 to 5 min after injection, the extracellular contrast reaches equilibrium with the concentration in the vascular system. This is known as the equilibrium phase. This unique blood supply to the liver is exploited by contrast imaging techniques because many mass lesions have characteristic patterns of appearance in the arterial, portal venous, and equilibrium phases. Newer contrast agents that are taken up by functioning hepatocytes and excreted into bile, such as disodium gadoxetate (Gd-EOB- DTPA; Eovist; Bayer Corporation, Pittsburgh, PA) and gadobenate dimeglumine (Gd-BOPTA; MultiHance; Bracco Diagnostics Inc., Princeton, NJ), provide further phenotypic characterization of liver masses and are particularly useful in the differentiation of adenomas from focal nodular hyperplasias (FNHs) and the diagnosis of HCC and metastases. The enhancement of hepatocytes with these hepatobiliary contrast agents in the hepatocyte or parenchymal phase typically peaks between 20 and 60 min after intravenous injection. Uptake of gadoxetate and gadobenate is believed to occur mainly through cell membrane proteins in the bile canaliculi and ducts, including organic anion transporting polypeptides and multidrug resistance protein. The expression of these proteins is usually suppressed in adenomas and HCCs and lack of the hepatocyte phase enhancement is useful in differentiating them from FNH.
Magnetic resonance elastography and acoustic radiation force impulse imaging are currently under investigation and may potentially be useful techniques in the characterization of liver masses.
Radiological findings may not always reflect true liver pathology. CT and MRI are instrumental in delineating intra- and extra-hepatic extent of disease [4, 7]. MRI can further improve diagnosis by defining features such as signal intensity characteristics, vascularity, stromal component and intra-lesional necrosis and hemorrhage [1]. Positron-emission tomography (PET) CT offers a greater sensitivity for residual and relapsed disease and may facilitate surgery. Radiological findings alone are useful in diagnosis in 58 % of cases, while in remainder histology is necessary [1].
Histological Diagnosis
Needle biopsies combined with histopathology and immunohistochemistry can be definitive for evaluating patients with discrete hepatic masses. However, liver biopsy poses a number of diagnostic challenges [3]. The incidence of complications after percutaneous liver biopsy reported in pediatric patients is 6.83 %, of which 2.4 % were major complications. Correlation with clinical, radiological, and cytological findings is helpful in arriving at the correct diagnosis and therefore increases overall accuracy and cost effectiveness of the procedure [10]. Common space occupying lesions of liver are tabulated in Table 2.
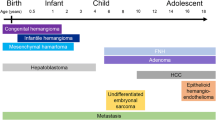
The imaging algorithms useful to diagnose hepatic lesions in children are given as Figs. 1 and 2 [7].
Hepatoblastoma: a & b Pre treatment T1 fat sat post intravenous gadolinium axial and coronal sequence of the liver show a large predominant non enhancing mass occupying the right lobe of liver and involving the right hepatic vein and right branch of portal vein. c & d Post treatment T1 fat sat post intravenous gadolinium axial and coronal sequences of the liver show more than 50 % reduction in the size of mass in the right lobe of liver and retraction from right hepatic vein and right branch of portal vein
Hemangioendothelioma: a T1 axial sequence shows multiple rounded and oval hypointense lesions in both lobes of liver. b STIR coronal sequence shows multiple iso - hypoechoic lesions in both lobes of liver. c T1 vibe fat sat axial sequence in arterial phase shows peripheral intense ring enhancement of the liver lesions. d T1 vibe fat sat axial sequence in portal venous phase shows variable fill in of contrast in the liver lesions
Hemangioendothelioma: a Colour Doppler ultrasound of the liver shows multiple oval iso to hyper echoic lesions with increased vascularity. b Angiogram of hepatic artery reveals increased vascular recruitment by the liver lesions. c Angiogram of hepatic artery following coil embolization reveals reduced vascularity by the liver lesions
Mesenchymal Hamartoma: a & b STIR axial and coronal sequences of liver demonstrate a large cyst occupying the right lobe of liver with incomplete internal septae. c & d T1 fat sat post intravenous gadolinium axial and coronal sequence of the liver show non-enhancing centre, enhancing peripheral rim and enhancing incomplete internal septae
Nodular regenerative hyperplasia: a T1 axial sequence shows round lesion with iso to hyper intense rim with hypo intense centre. b T2 axial sequence shows round lesion with iso to hypo intense rim with mildly hyperintense centre. c T1 VIBE axial fat sat post intravenous Gadolinium sequence arterial phase shows round lesion with enhancing rim and non-enhancing centre. d T1 VIBE axial fat sat post intravenous Gadolinium sequence late venous phase shows round lesion iso to hyper to the rest of liver due to complete fill in of contrast
The malignant liver lesions are enlisted in Table 3. Table 4 enlists classifications of the clinical categories of infantile hemangiomas of the liver. Table 5 provides list of benign lesions of the liver.
Conclusions
The diagnosis of pediatric liver lesions is made on the basis of clinical features, serum α-fetoprotein (AFP) level, age of the child, and imaging characteristics. The role of imaging is to determine the organ of origin and the character and extent of the lesion. Knowledge of pediatric liver diseases and their imaging appearances is essential in order to make an appropriate differential diagnosis. Challenges exist for the non-invasive detection and characterization of focal liver lesions (FLLs) in the pediatric population. The selection of the appropriate imaging test depends on a number of factors, such as:
-
(1)
Children require imaging strategies with a higher resolution due to smaller anatomic structures [9].
-
(2)
Children may be unable to tolerate or hold still for an imaging test, so they may require sedation or anesthesia [9].
-
(3)
The use of imaging tests with ionizing radiation should be minimized given that children are more sensitive to the long-term effects of radiation exposure than adults [9].
Differentiation of masses is still complex, and biopsy or resection for histological diagnosis sometimes becomes necessary. The incidence of complications after percutaneous liver biopsy in pediatric patients was 6.83 %, of which 2.4 % were major complications.
References
Dezsőfi A, McLin V, Hadzic N. Hepatic neoplasms in children: a focus on differential diagnosis. Clin Res Hepatol Gastroenterol. 2014;38:399–402.
Chung EM, Cube R, Lewis RB, Conran RM. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010;30:801–26.
Kochin IN, Miloh TA, Arnon R, Iyer KR, Suchy FJ, Kerkar N. Benign liver masses and lesions in children: 53 cases over 12 years. Isr Med Assoc J. 2011;3:542–7.
Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13:812–20.
von Schweinitz D. Management of liver tumors in childhood. Semin Pediatr Surg. 2006;15:17–24.
de Oliveria Andrade LJ, D’Oliveira A, Melo RC, De Souza EC, Costa Silva CA, Paraná R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1:33–7.
Jha P, Chawla SC, Tavri S, Patel C, Gooding C, Daldrup-Link H. Pediatric liver tumors--a pictorial review. Eur Radiol. 2009;19:209–19.
Das CJ, Dhingra S, Gupta AK, Iyer V, Agarwala S. Imaging of paediatric liver tumours with pathological correlation. Clin Radiol. 2009;64:1015–25.
Chiorean L, Cui XW, Tannapfel A, et al. Benign liver tumors in pediatric patients - review with emphasis on imaging features. World J Gastroenterol. 2015;21:8541–61.
Chhieng DC. Fine needle aspiration biopsy of liver - an update. World J Surg Oncol. 2004;2:5.
Meyers RL, Scaife ER. Benign liver and biliary tract masses in infants and toddlers. Semin Pediatr Surg. 2000;9:146–55.
Hegde SV, Dillman JR, Lopez MJ, Strouse PJ. Imaging of multifocal liver lesions in children and adolescents. Cancer Imaging. 2013;12:516–29.
Emre S1, McKenna GJ. Liver tumors in children. Pediatr Transplant. 2004;8:632–8.
Fernandez-Pineda I, Cabello-Laureano R. Differential diagnosis and management of liver tumors in infants. World J Hepatol. 2014;6:486–95.
Reynolds M. Current status of liver tumors in children. Semin Pediatr Surg. 2001;10:140–5.
Meyers RL. Tumors of the liver in children. Surg Oncol. 2007;16:195–203.
Chung EM, Lattin GE Jr, Cube R, et al. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics. 2011;31:483–507.
Helmberger TK, Ros PR, Mergo PJ, Tomczak R, Reiser MF. Pediatric liver neoplasms: a radiologic-pathologic correlation. Eur Radiol. 1999;9:1339–47.
Stocker JT. Hepatic tumors in children. Clin Liver Dis. 2001;5:259–81.
Isaacs Jr H. Fetal and neonatal hepatic tumors. J Pediatr Surg. 2007;42:1797–803.
Christison-Lagay ER, Burrows PE, Alomari A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–7; discussion 67–8.
Kassarjian A, Zurakowski D, Dubois J, Paltiel HJ, Fishman SJ, Burrows PE. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. AJR Am J Roentgenol. 2004;182:785–95.
Acknowledgments
Education Centre, Birmingham Children’s Hospital NHS Foundation Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Thyagarajan, M.S., Sharif, K. Space Occupying Lesions in the Liver. Indian J Pediatr 83, 1291–1302 (2016). https://doi.org/10.1007/s12098-016-2240-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-016-2240-x