Abstract
In developing countries, meconium aspiration syndrome (MAS) is an important cause of morbidity and mortality among neonates. The concepts of pathophysiology and management of meconium stained amniotic fluid (MSAF) and meconium aspiration syndrome have undergone tremendous change in recent years. Routine intranatal and postnatal endotracheal suctioning of meconium in vigorous infants is no longer recommended. Recent studies have challenged its role even in non-vigorous infants. Supportive therapy like oxygen supplementation, mechanical ventilation and intravenous fluids are the cornerstone in the management of meconium aspiration syndrome. Availability of surfactant, inhaled nitric oxide, high frequency ventilators and extracorporeal membrane oxygenation has made it possible to salvage more infants with meconium aspiration syndrome. In this review the authors have discussed the current concepts in the pathophysiology and management of MAS. Drugs in trials and future therapeutic targets are also discussed briefly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A neonate has meconium aspiration syndrome (MAS) if the triad of meconium stained amniotic fluid (MSAF), respiratory distress and typical radiological features is noted [1]. It accounts for 10 % of all causes of respiratory failure in neonates with mortality of 20 % in the developing countries [2]. Also, newborns exposed to meconium, are more likely to have complications like neonatal sepsis, seizures, neurologic impairment and prolonged NICU stay [3]. The severity of MAS as follows: (1) mild: neonate requires less than 40 % oxygen for less than 48 h; (2) moderate: neonate requires more than 40 % oxygen for more than 48 h with no air leak syndromes and (3) severe: neonate requires assisted ventilation for more than 48 h.
With changing concepts of pathophysiology, the intranatal and postnatal management of meconium stained amniotic fluid has undergone tremendous change in the last four decades. Routine intranatal and postnatal endotracheal suction of meconium in vigorous infants is no longer recommended [4]. Recent studies have challenged its role even in non-vigorous infants [5]. Supportive therapy like mechanical ventilation and intravenous fluids are the cornerstone in the management of MAS [6]. Availability of inhaled nitric oxide, high frequency ventilators and extracorporeal membrane oxygenation has made it possible to salvage more infants with MAS [1].
Pathophysiology of Meconium Passage and Meconium Aspiration Syndrome
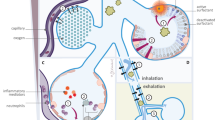
When a neonate aspirates meconium during intrauterine gasping or during initial breaths at birth, MAS ensues. Fetal hypoxic stress or vagal stimulation due to cord compression stimulates peristalsis in the colon [7]. The evidence is mounting for a chronic in-utero insult that may be more important for meconium passage as opposed to an acute peripartum event. Hypoxia also causes fetal gasping that results in meconium aspiration. Term and post-term fetuses are more likely to pass meconium in response to such a stress than preterms. Gastrointestinal maturation may be inadequate in preterms to be able to pass meconium; although, both the presence of meconium and active intestinal peristalsis have been reported as early as 8 wk of gestational age [7]. Erythropoietin levels were found to be increased in post-date fetuses and in fetuses who pass meconium at early gestation, possibly indicating chronic hypoxia contributing to the passage of meconium in-utero [8]. In-utero passage of meconium also increases the risk of intra-amniotic infection. Monen et al. suggested that MSAF should be considered as a symptom rather than a specific syndrome, becoming more frequent with increasing gestational age and which may be associated with increased incidence of infection or perinatal asphyxia [9].
The concept of primary and secondary MSAF was originally proposed by Meis et al. in 1982. Recently, there has been renewed interest in this theory [9]. MSAF is considered primary if there is meconium staining right from the time of rupture of membrane. If the amniotic fluid at the time of membrane rupture was clear but subsequently becomes meconium-stained, it is considered secondary. According to Hiersch et al., secondary MSAF reflects fetal distress while primary MSAF is a sign of fetal maturation [10].
Several mechanisms are involved in the pathophysiology of MAS including acute airway obstruction, surfactant dysfunction, chemical pneumonitis and direct toxic effect, persistent pulmonary hypertension of the newborn (PPHN) with right-to-left shunt and secondary infection [7, 11]. Airway obstruction after intrapartum ingestion of meconium was considered to be the primary mechanism of MAS in the past [11]. Meconium may cause partial or complete airway obstruction. With the breathing movements, meconium migrates from proximal to distal airways. In these small distal airways, meconium particles cause obstruction, atelectasis and hyperinflation leading to ventilation/perfusion (V/Q) mismatch and air leak. However, many authors argue that the pathophysiology of MAS involves more complex process than the mere aspiration of meconium and presence of MSAF is a marker of infants at risk than the cause for MAS. Surfactant dysfunction is an alternative mechanism for meconium induced atelectasis. Inflammatory mediators and free radicals induced by meconium may also injure the alveoli [12].
Meconium may have a direct toxic effect on the alveoli. Within few hours of meconium aspiration, neutrophils and macrophages are present in the alveoli and airways. Cytokines including Tumor necrosis factor-α, Interleukin-1β and Interleukin-8, released by these inflammatory cells, cause direct injury to the lung parenchyma and vascular endothelium. This leads on to capillary leak, toxic pneumonitis and hemorrhagic pulmonary edema. The cytokines and free radicals injure airway epithelial cells and lead to apoptosis mediated cell death. There is a hypothesis that the components of innate immunity, the toll-like receptors and the complement system are involved in lung dysfunction and systemic inflammatory response in MAS [13]. In infants with MAS, 20 % to 40 % develop significant pulmonary hypertension. PPHN may also be contributed by asphyxia, which has a significant association with MAS [7].
Many components of meconium, like bile acids can cause direct injury to the umbilical cord vessels and amniotic membranes. This may also lead to fetal tissue and organ damage [14]. Tissue injury by meconium may range from only mild inflammation in the lung, placental membranes and chorionic plate to severe focal injury to the umbilical vessel. Meconium induced umbilical vessel constriction, vessel necrosis, and production of thrombi may lead to severe hypoxic-ischemic injury.
Meconium is a rich medium that can potentially enhance bacterial growth in vitro. Histological features of pneumonia are frequently seen at autopsy in MAS affected neonates treated with mechanical ventilation [15]. There is increased risk of positive amniotic fluid cultures and clinical chorioamnionitis in infants with MSAF. It is unclear if these infections are the consequence of MSAF, or whether intra-uterine infection is a predisposing factor for MSAF [3]. A clear relationship between MSAF and sepsis has not been demonstrated. Moreover, prophylactic antibiotics are not found to be beneficial in MAS [16].
Non-MAS respiratory disorders are found to be more common in neonates born through MSAF compared to those delivered through clear liquor. The risk factors for these disorders are virtually identical to those for MAS. Wiswell et al. suggested that, all these disorders represent the overall spectrum of MAS rather than distinct entities [17].
Prevention of Meconium Aspiration Syndrome
The reduction in the incidence of MAS over the last two decades is attributed to the reduction in post-term delivery by elective induction of pregnancies more than 41 wk gestation, aggressive management of fetal distress, and decreased incidence of birth asphyxia [11]. A skilled resuscitation team must be present in all deliveries complicated by MSAF [6]. Elective induction of labor at 41 wk gestation is the most logical step towards prevention of maturation induced intra-uterine meconium passage and hence, MAS. Intrapartum amnioinfusion with saline has been used to dilute the thick meconium and this procedure may prevent umbilical cord compression especially in pregnancies complicated by oligohydramnios with MSAF. It may also lead to reduced fetal hypoxia-induced gasping and subsequent meconium aspiration [11]. Cochrane meta-analysis of 14 studies by Hofmeyr et al. did not show improvement in perinatal outcome, in resource rich settings where standard peripartum monitoring facilities are available [18, 19]. It is not known whether the improved outcome is due to dilution of meconium or increased amount of amniotic fluid in oligohydramnios [19]. The lack of benefit in resource rich settings may be due to the fact that majority of meconium aspiration occurs before labor. Chorioamnionitis, premature rupture of membranes, placental abruption, preterm labor, cord hemorrhage, cord prolapse and amniotic fluid embolism and maternal death are known complications of amnioinfusion [18, 20].
Based on the concept that meconium aspiration is in-utero rather than a postpartum event, in-utero endoscopic suctioning of meconium has been tried when thick meconium was detected upon rupture of the membranes. However, the usefulness of this intervention has not been tested in any other study.
Two large randomized controlled trials in the last decade by Vain et al. [21] and Wiswell et al. [17], brought landmark changes in the management of meconium-stained infants. Routine intrapartum oropharyngeal suction and immediate postnatal suction in vigorous babies were abandoned in NRP 2005 guidelines [4]. A recent study by Nangia et al. showed that even in developing countries with poor ante-partum/intra-partum fetal surveillance and the late arrival of mothers with prolonged fetal distress, intrapartum suction does not reduce the incidence or severity of MAS [22]. The change in guidelines in vigorous infants has not led to increase in the incidence of MAS. The primary reason that endotracheal suction is not able to reduce the risk of MAS significantly could be because of both meconium passage and aspiration occur in-utero and the tracheal suctioning is unlikely to benefit as meconium already is in the distal lung at the time of birth.
Postnatal endotracheal suction in non-vigorous meconium-stained infants is a subject of debate. Although these infants are at a high risk of MAS, repeated tracheal suction has not led to the reduction in incidence of MAS in the past [4]. NRP 2010 recommends routine postnatal suction in non-vigorous meconium-stained infants due to lack of evidence on the benefit or harm of the procedure. In a recent randomized trial in authors’ center, they did not find any benefit of tracheal suction in preventing MAS, mortality, asphyxia, the need for and duration of mechanical ventilation, duration of NICU stay, shock, secondary sepsis and neurodevelopmental outcome at 9 mo of age [5]. However, the study was underpowered to find the differences in the outcomes other than the risk of MAS. Further studies are warranted to assess the role of tracheal suction in non-vigorous infants.
Treatment of Meconium Aspiration Syndrome
Supportive Therapy
All the infants with MAS should be admitted in a level III NICU and monitored using pulse oximetry. Frequent blood gas analysis, preferably using an indwelling arterial catheter may be required. Minimal stimulation, sedation and analgesia are some of the important strategies to decrease pain and discomfort which in turn may lead to hypoxia and right-to-left shunting. It is prudent to cover the eyes of the infant and maintain noise free environment for preventing PPHN. Morphine or fentanyl is often used to optimize gas exchange. This can also help in avoiding ventilator patient asynchrony, reflex catecholamine release and worsening pulmonary vascular resistance. Neuromuscular blockade using pancuronium or vecuronium are believed to decrease agitation and hence, hypoxic episodes in ventilated infants. The other potential benefits include improved oxygenation, decreased oxygen consumption and decreased accidental extubations. On the other hand, paralysis may promote atelectasis and cause ventilation-perfusion mismatch. This may increase the risk of mortality among infants with MAS. The use of neuromuscular blockade in MAS is still controversial and should be reserved for infants who cannot be managed with sedatives alone. Maintaining normothermia and correction of metabolic abnormalities including hypoglycemia, hypocalcemia, acidosis and polycythemia are also crucial.
Nasogastric Aspiration
Prophylactic nasogastric suction before the first feed has been suggested to reduce the risk of feed intolerance and secondary meconium aspiration in meconium-stained babies. In various randomized trials, there is no clear benefit of this practice in reducing both the outcomes [23–26]. However, a recent meta-analysis of 6 trials by Deshmukh et al. suggested that prophylactic lavage might reduce the risk of feed intolerance in meconium stained infants by 30 % [27]. But, they interpreted their results with caution due to several limitations of the study which included the inclusion of trials with high risk of bias, lack of a standard definition of feed intolerance, subjectivity in outcome assessment and lack of data on confounding factors (e.g., sepsis, swallowed maternal blood). Statistical significance is lost after using random-effects model and after sensitivity analysis excluding high risk of bias studies. The role of the procedure in preventing secondary meconium aspiration was not assessed. A large randomized controlled trial is needed to address these issues.
Respiratory Support
Depending on the severity, respiratory support may vary. Some infants may require only oxygen by hood. However, 40 % of infants require mechanical ventilation and 10 % require continuous positive airway pressure [17]. Usually the target oxygen saturation is between 90 and 95 %. Target PaO2 may be as high as 90 mmHg as the risk of retinopathy and lung toxicity is less in term infants, and intermittent hypoxemia may result in the development or worsening of PPHN [28].
Mechanical Ventilation
No other condition in newborns poses as much challenge for mechanical ventilation than MAS. These infants have lungs with areas of atelectasis co-existing with areas of hyperinflation associated with ventilation-perfusion mismatch and airway compromise. Synchronized intermittent mandatory ventilation or assist/control with adequate sedation is preferred. The aim of mechanical ventilation should be to improve oxygenation and simultaneously minimize barotrauma. As in any other condition with respiratory failure, infants with severe hypoxemia (PaO2 < 50 mmHg) or hypercarbia (PaCO2 > 60 mmHg) or acidosis (pH less than 7.25) with FiO2 > 0.6 should be intubated and ventilated. Ventilator settings and blood gas targets depend upon presence or absence of PPHN. Mild hypoxemia may be permitted in MAS without PPHN [28]. In MAS complicated by PPHN, higher oxygenation targets (PaO2 80–100 mmHg) with saturation target 95 to 99 % and mild hypocapnea (PaCO2 30–35 mmHg) with pH of 7.4–7.5 are desired. This may be achieved with mild hyperventilation and higher FiO2. But the caretaker must be wary of the risk of cerebral vasoconstriction and ischemia leading to long-term neurologic morbidity, hearing deficits as well as air leaks with hyperventilation and hypocapnea. In such situations, other modalities like inhaled nitric oxide (discussed later) high frequency ventilation (HFV) should be considered early. Theoretically, HFV is expected to reduce barotrauma and air leak syndromes in MAS, but evidence from animal and clinical models is conflicting. Partial liquid ventilation was found to be a better in delivering surfactant in an adult rat model of MAS compared to conventional mechanical ventilation [29].
Surfactant Therapy
The Canadian Pediatric Society recommends exogenous surfactant for all intubated infants with MAS requiring FiO2 ≥ 50 %. Surfactant may be administered as bolus therapy or bronchoalveolar lavage. A meta-analysis has shown that bolus surfactant therapy reduced the severity of illness and requirement of ECMO [30]. However, mortality, hospital stay, duration of ventilation, oxygen use, pneumothorax, pulmonary interstitial emphysema, or chronic lung disease were not altered by surfactant therapy. Clinical trial of surfactant lavage found no difference between lavage infants and controls in terms of ECMO requirements, air leak or duration of ventilation [31]. Moreover, lavage procedure was often halted due to hypotension or episodes of hypoxemia [6].
Steroids
According to a Cochrane meta-analysis, systemic steroids did not reduce mortality among infants with MAS [32]. Few Indian studies have shown decrease in the duration of oxygen therapy and hospital stay with steroid therapy [33]. Steroids may benefit those infants with severe MAS who have lung edema, pulmonary vasoconstriction and inflammation. As of now, routine steroid therapy for the management of MAS is not recommended. A large randomized controlled trial on steroids in MAS is indeed the need of the hour [34].
Antibiotics
The presence of meconium has been shown to increase the positivity of cultures from amniotic fluid. Moreover, secondary pneumonia has been proposed as a mechanism in MAS [15]. However, the relationship of MSAF and sepsis has not been clearly documented. Studies have shown that routine antibiotic prophylaxis is not beneficial in MAS for infants without other risk factors for sepsis [35, 36]. The clinical course and outcome related to infection in MAS were not affected by antibiotic therapy. The role of antibiotics in the management of MAS needs to be re-evaluated.
Inhaled Nitric Oxide (iNO)
PPHN is a common complication and is the leading cause of death in severe MAS [13]. Inhaled nitric oxide acts on vascular smooth muscle causing selective pulmonary vasodilation [6]. It is an ideal agent in PPHN as it causes pulmonary vasodilatation in ventilated areas of lung, decreasing the ventilation-perfusion mismatch and thus, improves oxygenation. As it does not affect systemic vascular resistance while improving pulmonary blood flow, the right to left shunt is reduced. Randomized controlled trials have shown that iNO therapy decreases the need for ECMO and mortality in full-term and near-term neonates with hypoxic respiratory failure and PPHN [1]. An oxygenation index of 25 may be the optimal time of initiating iNO [37]. A synergestic combination of HFV and iNO improved oxygenation in some infants with severe PPHN. Better lung inflation during HFV can decrease intrapulmonary shunting and improve iNO delivery to the pulmonary circulation, thus augmenting the response to iNO.
A significant proportion of infants with PPHN (30–50 %) do not respond to iNO therapy. Also, the set up for iNO involves high cost and is not freely available in the developing countries. Phosphodiesterase-5 inhibitors including sildenafil, milrinone and dipyridamole may also be tried in infants who do not respond to iNO [37]. Prostacyclin, tolazoline, Magnesium sulfate, L-Arginine, free radical scavengers (e.g., superoxide dismutase) and endothelin antagonist like bosentan have been tried.
Extracorporeal Membrane Oxygenation (ECMO)
Infants with severe MAS and refractory respiratory failure have been treated with ECMO. The commonest indication of ECMO in newborns is MAS. Up to 35 % of the infant population who require ECMO is due to MAS [38]. When treated with ECMO, infants with MAS have a high survival rate approaching almost 95 %. Availability of iNO and HFV has led to reduced use of ECMO in MAS [1, 6].
The Future
Current therapies for MAS are supportive in nature and do not address the lung parenchymal injury due to meconium. Once meconium has crossed the level of vocal cords and reached the lung tissue, it is very difficult to prevent MAS. The oxidative stress is suggested to cause surfactant inactivation in MAS. Hence, antioxidants like N-acetylcysteine (NAC) are tried in experimental animals with MAS. NAC may also reduce the viscosity of meconium by breaking disulphide bonds between protein molecules [39]. However, NAC alone had only mild therapeutic effect on MAS in animal studies. Combination of NAC with surfactants showed enhanced therapeutic benefit than either treatment alone [40]. A protease inhibitor cocktail was noted to prevent the cell detachment induced by meconium. Hence, fetal pancreatic enzymes may be handy in treating meconium induced lung injury [41].
Conclusions
Due to improvements in both obstetric and neonatal management strategies, the morbidity and mortality associated with MAS has reduced considerably in the west. However, MAS continues to be a major neonatal problem in the developing countries. The pathophysiology of MAS is complex and is characterized by airway obstruction, inactivation of surfactant, chemical pneumonitis, and PPHN. Elective labor induction for select pregnancies and amnioinfusion are key preventive strategies against MAS. Postnatal endotracheal suctioning to clear meconium is of no benefit in vigorous and needs further evaluation in non-vigorous infants. Supportive care is the cornerstone of management of neonates with MAS.
References
Swarnam K, Soraisham AS, Sivanandan S. Advances in the management of meconium aspiration syndrome. Int J Pediatr. 2012;2012:359571.
Singh BS, Clark RH, Powers RJ, Spitzer AR. Meconium aspiration syndrome remains a significant problem in the NICU: outcomes and treatment patterns in term neonates admitted for intensive care during a ten-year period. J Perinatol. 2009;29:497–503.
Hutton EK, Thorpe J. Consequences of meconium stained amniotic fluid: what does the evidence tell us? Early Hum Dev. 2014;90:333–9.
Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S909–19.
Chettri S, Adhisivam B, Bhat BV. Endotracheal suction for non-vigorous neonates born through meconium stained amniotic fluid: a randomized controlled trial. J Pediatr. 2015;166:1208–13.
Walsh MC, Fanaroff JM. Meconium stained fluid: approach to the mother and the baby. Clin Perinatol. 2007;34:653–65.
Poggi SH, Ghidini A. Pathophysiology of meconium passage into the amniotic fluid. Early Hum Dev. 2009;85:607–10.
Liu WF, Harrington T. Delivery room risk factors for meconium aspiration syndrome. Am J Perinatol. 2002;19:367–78.
Monen L, Hasaart TH, Kuppens SM. The aetiology of meconium-stained amniotic fluid: pathologic hypoxia or physiologic foetal ripening? (Review). Early Hum Dev. 2014;90:325–8.
Hiersch L, Melamed N, Rosen H, Peled Y, Wiznitzer A, Yogev Y. New onset of meconium during labor versus primary meconium-stained amniotic fluid - is there a difference in pregnancy outcome? J Matern Fetal Neonatal Med. 2013;27:1361–7.
Vain NE, Szyld EG, Prudent LM, Aguilar AM. What (not) to do at and after delivery? Prevention and management of meconium aspiration syndrome. Early Hum Dev. 2009;85:621–6.
Van Ierland Y, de Beaufort AJ. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum Dev. 2009;85:617–20.
Lindenskov PH, Castellheim A, Saugstad OD, Mollnes TE. Meconium aspiration syndrome: possible pathophysiological mechanisms and future potential therapies. Neonatology. 2015;107:225–30.
Ahanya SN, Lakshmanan J, Morgan BL, Ross MG. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv. 2005;60:45–56.
Yeh TF. Core concepts: meconium aspiration syndrome: pathogenesis and current management. Neo Rev. 2010;11:e503–12.
Siriwachirachai T, Sangkomkamhang US, Lumbiganon P, Laopaiboon M. Antibiotics for meconium-stained amniotic fluid in labour for preventing maternal and neonatal infections. Cochrane Database Syst Rev. 2014;11:CD007772.
Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000;105:1–7.
Dad N, Abushama M, Konje JC, Ahmed B. What is the role of amnioinfusion in modern day obstetrics? J Matern Fetal Neonatal Med. 2015;28:1–13.
Hofmeyr GJ, Xu H, Eke AC. Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev. 2014;1:CD000014.
Gelfand SL, Fanaroff JM, Walsh MC. Controversies in the treatment of meconium aspiration syndrome. Clin Perinatol. 2004;31:445–52.
Vain NE, Szyld EG, Prudent LM, Wiswell TE, Aguilar AM, Vivas NI. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597–602.
Nangia S, Pal MM, Saili A, Gupta U. Effect of intrapartum oropharyngeal (IP-OP) suction on meconium aspiration syndrome in developing country: a RCT. Resuscitation. 2015;97:83–7.
Sharma P, Nangia S, Tiwari S, Goel A, Singla B, Saili A. Gastric lavage for prevention of feeding problems in neonates with meconium-stained amniotic fluid: a randomised controlled trial. Paediatr Int Child Health. 2014;34:115–9.
Garg J, Masand R, Tomar BS. Utility of gastric lavage in vigorous neonates delivered with meconium stained liquor: a randomized controlled trial. Int J Pediatr. 2014;2014:204807.
Mehta R. Gastric lavage in vigorous neonates born with meconium stained amniotic fluid. Indian J Pediatr. 2013;80:252.
Ameta G, Upadhyay A, Gothwal S, Singh K, Dubey K, Gupta A. Role of gastric lavage in vigorous neonates born with meconium stained amniotic fluid. Indian J Pediatr. 2013;80:195–8.
Deshmukh M, Balasubramanian H, Rao S, Patole S. Effect of gastric lavage on feeding in neonates born through meconium-stained liquor: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2015;100:F394–9.
Goldsmith JP. Continuous positive airway pressure and conventional mechanical ventilation in the treatment of meconium aspiration syndrome. J Perinatol. 2008;28:S49–55.
Chappell SE, Wolfson MR, Shaffer TH. A comparison of surfactant delivery with conventional mechanical ventilation and partial liquid ventilation in meconium aspiration injury. Respir Med. 2001;95:612–7.
El Shahed AI, Dargaville P, Ohlsson A, Soll RF. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database Syst Rev. 2007:CD002054.
Dargaville PA, Copnell B, Mills JF, et al. Randomized controlled trial of lung lavage with dilute surfactant for meconium aspiration syndrome. J Pediatr. 2011;158:383–9.
Ward M, Sinn J. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database Syst Rev. 2003:CD003485.
Garg N, Choudhary M, Sharma D, Dabi D, Choudhary JS, Choudhary SK. The role of early inhaled budesonide therapy in meconium aspiration in term newborns: a randomized control study. J Matern Fetal Neonatal Med. 2016;29:36–40.
Mokra D, Mokry J. Glucocorticoids in the treatment of neonatal meconium aspiration syndrome. Eur J Pediatr. 2011;170:1495–505.
Goel A, Nangia S, Saili A, Garg A, Sharma S, Randhawa VS. Role of prophylactic antibiotics in neonates born through meconium-stained amniotic fluid (MSAF)–a randomized controlled trial. Eur J Pediatr. 2015;174:237–43.
Basu S, Kumar A, Bhatia BD. Role of antibiotics in meconium aspiration syndrome. Ann Trop Paediatr. 2007;27:107–13.
Konduri GG, Solimano A, Sokol GM, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004;113:559–64.
Short BL. Extracorporeal membrane oxygenation: use in meconium aspiration syndrome. J Perinatol. 2008;28:S79–83.
Ivanov VA. Meconium aspiration syndrome treatment - new approaches using old drugs. Med Hypotheses. 2006;66:808–10.
Kopincova J, Mokra D, Mikolka P, Kolomaznik M, Calkovska A. N-acetylcysteine advancement of surfactant therapy in experimental meconium aspiration syndrome: possible mechanisms. Physiol Res. 2014;63:S629–42.
Ivanov VA, Gewolb IH, Uhal BD. A new look at the pathogenesis of the meconium aspiration syndrome: a role for fetal pancreatic proteolytic enzymes in epithelial cell detachment. Pediatr Res. 2010;68:221–4.
Contributions
SC did the literature search and prepared the initial draft. BVB and AB edited the manuscript. BVB will act as guarantor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Chettri, S., Bhat, B.V. & Adhisivam, B. Current Concepts in the Management of Meconium Aspiration Syndrome. Indian J Pediatr 83, 1125–1130 (2016). https://doi.org/10.1007/s12098-016-2128-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-016-2128-9