Abstract
Objective
To investigate the efficacy of probiotics added to oral rehydration solution and zinc in the treatment of acute infectious diarrhea in Indonesian children.
Methods
A prospective randomized double blind placebo-controlled trial was performed to test the efficacy of a probiotic food supplement in 112 children in the Kenari subdistrict, central Jakarta, aged 6–36 mo with acute infectious diarrhea and moderate dehydration. The supplemented group was given standard therapy (oral rehydration solution and zinc) and the probiotic strains Lactobacillus (L.) rhamnosus R0011 1.9 × 109 colony forming units (cfu) and L. acidophilus R0052 0.1 × 109 cfu/d for 7 d, while the control group was given standard therapy and placebo.
Results
Median duration of diarrhea was 68.5 h (range 13–165) in the supplemented and 61.5 h (range 21–166) in the control group (P = 0.596). Median daily frequency of defecation until diarrhea stopped was 5.0 in the supplemented vs. 5.5 in the control group (P = 0.795).
Conclusions
This probiotic food supplement tested did not reduce the duration of acute infectious diarrhea as compared to oral rehydration and zinc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute infectious gastroenteritis (GE) is still an important health problem in developing countries. Diarrhea is defined as an increase in the frequency, water content and volume of stools. The 2004 World Health Organization (WHO)/UNICEF report estimates an incidence of 1.6 million deaths in 2002 of which 15 % can be attributed to diarrhea [1].
Oral rehydration solution (ORS) is the cornerstone of management of dehydration [2]. Education of parents about hand hygiene, safe weaning and drinking water etc. can help in reducing the incidence [2]. The WHO recommends ORS supplemented with zinc in the treatment of acute infectious GE in children in developing countries [1, 3]. The usefulness of this recommendation was confirmed in a meta-analysis [4]. Zinc has been shown to reduce stool output, duration and severity of diarrhea [5].
Probiotics are living microorganisms that survive in the gastrointestinal (GI) tract and, when ingested in an appropriate amount, bring a health benefit to the host. The European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) recognized the benefit of some strains, but the WHO does not (yet) recommend probiotics [6, 7]. A review on the efficacy of probiotics and zinc in acute GE suggested that data on the effect of a combined administration of zinc and probiotics are needed [5]. This study, performed with one of the popular probiotic food supplements in Indonesia evaluated whether supplementation of two probiotic strains to standard therapy (ORS and zinc) has a beneficial effect on the duration of acute diarrhea and the frequency of defecation.
Material and Methods
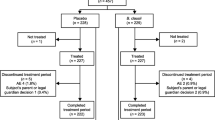
A double blind, placebo controlled, randomized prospective clinical trial was performed in the Kenari sub district, central Jakarta, between January through May 2012. Local health care volunteers informed the parents about the study and proposed to bring their children (6–36 mo) to an outpatient clinic. The minimum level of parental education was junior high school. The patients were evaluated for their eligibility to be included in the study.
The inclusion criteria were acute diarrhea, defined as (semi-) watery stools according to Bristol criteria > type 4 (Table 1) lasting for less than or equal to 48 h with mild to moderate dehydration [9, 10]. Diarrhea was considered cured when stool consistency was ≤4 regarding Bristol criteria and never >4 during 24 h. Dehydration was evaluated on clinical grounds and estimated weight loss. Severe dehydration was defined as a capillary refill of >4 s. Exclusion criteria included malnutrition (weight <P3), administration of zinc, chronic conditions (such as known chronic intestinal disease such as celiac disease, cystic fibrosis, food allergy, immune deficiency, inflammatory bowel disease, GI malformations, abnormal GI motility) and acute conditions (antibiotic treatment during the previous 7 d, macroscopic blood in the feces, use of probiotics except for the probiotics present in infant formula).
To obtain the required power (95 %, type 1 error = 0.05, two tailed test) 45 patients were needed in each group, assuming a mean difference in diarrhea duration of 24 h between both groups with a SD of 30 h, based on similar trials and a Cochrane review [11, 12]. The Cochrane review analyzed 63 studies (56 paediatric trials) including 8,014 participants, and concluded that the average effect was a decrease of the mean duration of diarrhea with 24.76 h (95 % confidence interval 15.9 to 33.6 h; n = 4,555, trials = 35) [12]. Considering an expected drop-out rate of 10 to 30 %, 112 children were included.
Subjects were divided in two groups by randomization using an envelope system, prepared by the Faculty of Medicine Pharmacy. Patients were enroled according to a computer-determined allocation. The sequence was concealed in an envelope and “the next” neutral envelope was opened when the next patient was included. The placebo was packed in identical capsules as the probiotic, which was provided by the manufacturer. Each group consisted of 56 subjects. Patients were given ORS (Indoralite®) at libitum and 20 mg zincsulphate/d during 10 d and the probiotic or placebo capsules for 7 d. The probiotic strains were Lactobacillus (L.) rhamnosus R0011 1.9 × 109 and L. acidophilus R0052 0.1 × 109 cfu/d (Lacidofil®, Institute Rossel Inc, Canada, Dexa Meda, Indonesia). All the products (probiotics, oral rehydration, zinc) were provided for free by the producing companies. The capsules with the probiotic or the placebo were diluted in a table spoon of water. After 4 to 6 h of rehydration with ORS exclusively, children were fed again according to their normal intake.
Parents were shown how to give the drugs, to record stool frequency and adverse events in a diary. One of the researchers phoned the parents daily to ensure the condition of all patients and to check that all parameters were recorded. The researcher examined all the children when according to the information diarrhea had resolved or on day 7. Recovery was defined as first stools with normal consistency (Bristol scale ≤4.0) [11] and no stool with Bristol scale >4.0 during 24 h.
The study was approved by the local ethical committee of Cipto Mangunkusumo Hospital, Jakarta, Faculty of Medicine, University of Indonesia. All statistical analyses were conducted using SPSS version 17.0 for Windows. Variables were described as percent, mean and standard deviation or median and range as appropriate. Difference between two groups was assessed by using Mann–Whitney U test. P values < 0.05 were considered statistically significant. The analysis was based on intention to treat.
Results
A total of 150 consecutive subjects were eligible; 38 did not meet the inclusion criteria (n:25; 13 in probiotic group; 12 in placebo group) because of inappropriate age (n:7): too young or too old (n:18), or refusal of the parents to participate (n:13; 6 in probiotic group; 7 in placebo group). One hundred and twelve children were included (56 in each group; patient characteristics: Table 2). All children belonged to the middle-class and none of the children was malnourished. None of the subjects was severely dehydrated. Ten children (5 in each group) refused to drink ORS. Four subjects did vomit after ingestion of zinc on the first day of treatment. All patients were compliant with the protocol; there was no drop-out.
The difference in outcome between both groups was statistically not significant (Table 3). Compliance was checked with a daily telephone contact, and parents had to return study medication at the end of the study. Daily frequency of defecation (at inclusion) was 5.0 and 5.5/d (stool consistency Bristol criteria >4). Recovery was defined as the first stools with normal consistency (Bristol scale ≤4.0) and no stool with Bristol scale >4.0 during 24 h. No adverse effect was reported.
Discussion
This study found that the probiotic strains tested did not shorten the duration of diarrhea in comparison to standard therapy with ORS and zinc. The frequency of defecation was slightly, but not statistically nor clinically significant, reduced. This result is in contrast with studies reporting that (other) probiotic strains did shorten the duration of diarrhea with 7.2 to 2.05 h compared to ORS and zinc [13, 14].
Probiotics may be beneficial in acute GE for various reasons, such as a direct antagonistic effect against pathogens inhibiting adhesion to the mucosa, production of mucin, bacteriocins or other antimicrobial substances [5].
Zinc is part of many metabolic processes and immune functions. Zinc stimulates the regeneration of the mucosa. Zinc also decreases ion secretion and nitric oxide synthesis. Zinc decreases intestinal permeability. An important effect is also the appetite stimulation [5, 15]. But according to a recent Indian trial, zinc failed to decrease stool output or shorten recovering time [16]. But, in an Italian study, zinc (and prebiotics) added to ORS was shown to improve outcome [17]. The majority of data suggest that children in developing countries older than 6 mo may benefit from the administration of zinc in the treatment of acute GE [6].
The combination of zinc and probiotics decreases the severity and duration of diarrhea [2]. The reduction in frequency of defecation (0.1–1 time less) reported in the present trial is in line with the data from literature [14]. Streptococcus Thermophilus, Bifidobacterium lactis and L. acidophilus (2 × 109 of each strain) and zinc (10 mg/d) and 0.3 g fructo-oligosaccharides reduced the duration of diarrhea with half a day (1.41 ± 0.71 vs. 1.96 ± 1.24 day) in infants 6–12 mo old in Galilee (Israel) [13]. Agustina et al. showed in an Indonesian population that L. reuteri DSM17938 may prevent diarrhea, especially in children with a poor nutritional status [18]. L. casei CRL431, did not prevent diarrhea [18]. None of the interventions affected the incidence of respiratory tract infections [18]. The major difference between the study by Agustina and the present study was that the authors did a therapeutic intervention trial while Agustina did a prevention study.
Several factors such as recall bias, compliance to therapy and causative agents might interfere with the present study results. Also, the authors cannot exclude a type II error (false negative), because of the relative small sample size. Because of restricted budget, the authors could not perform an independent quality analysis of the tested probiotic product (the vast majority of similar trials did also not test this). The authors did not identify the pathogens that may have caused diarrhea. Stool cultures were not performed, as is the case in many studies with prebiotics and probiotics [11, 18]. According to the evidence-based guidelines of ESPGHAN and the European Society of Infectious Disease it is not recommended to perform stool cultures for acute GE in primary health care [7]. Moreover, since the present study was not funded (except for the free medication), there was no budget to perform stool analyses.
Meta-analyses report significant differences in effectiveness for different probiotic species and strains such as Saccharomyces (S.) boulardii, Clostridium butyricum, Bifidobacterium (B.) lactis, L. rhamnosus GG, other Lactobacilli species and probiotic mixtures [12, 19]. A recent Indian study showed that S. boulardii administration did result in a significant reduction of the duration of diarrhea: 52.08 ± 24.57 vs. 64.04 ± 30.43 h [20]. The time of appearance of the first semi formed stool in the S. Boulardii group (39.48 ± 23.09 h) was significantly (95 % CI −25.4 to −3.87) shorter than the placebo group (54.13 ± 28.21 h) [20]. Bifilac (Streptococcus faecalis, Clostridium butyricum, Bacillus mesentericus, Lactobacillus sporogenes) resulted in a clinical and statistically significant reduction in the number of stools per day, the duration of diarrhea, recovery from dehydration, duration and volume of oral rehydration needed, duration of intravenous rehydration and rotaviral shedding [21]. However, the scientific design of this study was questioned [22].
Negative results were also shown with L. casei strain GG (LGG) and ORS without zinc [23]. Duration of diarrhea was 8.1 h longer and total fecal output was 52.8 mL/kg more in the supplemented compared to the control group, although this difference was not statistically significant [23]. Positive effects of LGG have been reported in several other studies, the negative result in this study may be related to a transient lactose malabsorption, which is common in acute diarrhea [23].
The authors administered L. acidophilus Rosell-52 and L. rhamnosus Rosell-11. The same combination of probiotics but without zinc supplementation showed a reduction in the duration of diarrhea [24, 25]. Since the results of the present study are negative, it may be that the efficacy of zinc minimizes the additional benefit of probiotics. Dalgic et al. reported in a study comparing different treatment options (S. boulardii alone; zinc alone; lactose-free formula alone; S. boulardii plus zinc; S. boulardii plus lactose-free formula; zinc plus lactose-free formula; S. boulardii plus zinc plus lactose-free formula; only oral and/or parenteral rehydration) that only “zinc alone” and “zinc with S. boulardii” reduced the duration of diarrhea [14]. The present study may have turned out negative because of inefficacy of the probiotic tested (but another study reported a positive effect with the same probiotic combination) or because the additional zinc overruled the efficacy of the probiotics. This aspect needs to be further investigated.
Another aspect regards the dosage of the probiotic strains. The authors used 2 × 109 cfu given once daily for seven days. A meta-analysis showed no significant difference in efficacy (79 % vs. 75 %) in children with acute diarrhea who were given 1 × 107 to 6 × 109 cfu/d compared to a dose of 1 × 1010 to 2 × 1011 cfu/d [19]. A study with the same combination of strains as in the present study did report that one capsule daily shortened the duration of acute diarrhea [25]. There are no recommendations regarding the optimal duration of probiotic supplementation. Depending on the indication, the duration of administration reported varies between 2 d and 6 mo [19].
Temperature during storage of probiotics is another important aspect, especially in tropical countries [26]. Environmental temperature and gastric conditions determine the viability of probiotics [27]. The label on many probiotics mentions that storage needs to be in a dry, cool place by preference at 4 °C. In the present study, the probiotics were stored at room temperature in a dry place in the house. Storage of the probiotics in the patient’s home for 7 d should not undermine the viabilityof the bacteria in the capsule. L. acidophilus and B. bifidum stored for 1 mo at 28–32 °C or stored at temperature of 4 °C have the same efficacy in reducing the duration of diarrhea: 34.1 vs. 34.8 h, while the duration was 58 h for the placebo (p < 0.05) [28]. Stability of the strains in the present study has been tested for different temperatures: storage at a temperature of 25 °C for 4 wk reduced the cfu with 13 % and storage for 12 mo at 25 °C resulted in a reduction with 27 % [29]. The number of cfu was unchanged if storage was for 1 wk only [29].
Therapeutic strategies need to be assessed in different settings and pharmaco-economic analyses based on country-specific data are needed [30]. Clinical practice should implement guidelines adapted to a local level, taking into account cost [30].
Conclusions
The probiotic mixture tested failed to reduce the duration of acute infectious diarrhea. These results suggest that there is no additional advantage of the probiotic product studied to the effect of zinc and ORS. This study illustrates that each probiotic product should be clinically tested.
References
The United Nations Children’s Fund/World Health Organization. WHO/UNICEF joint statement: clinical management of acute diarrhea. 2004. [cited 2008 April 1]. Available from http://www.mostproject.org/ZINC/WHO-UNICEF%20Statement.pdf
Dekate P, Jayashree M, Singhi SC. Management of acute diarrhea in emergency room. Indian J Pediatr. 2013;80:235–46.
Hoque KM, Binder HJ. Zinc in the treatment of acute diarrhea: Current status and assessment. Gastroenterology. 2006;130:2201–5.
Patro B, Golicki D, Szajewska H. Meta-analysis: Zinc supplementation for acute gastroenteritis in children. Aliment Pharmacol Ther. 2008;28:713–23.
Salvatore S, Hauser B, Devreker T, Vieira MC, Luini C, Arrigo S, et al. Probiotics and zinc in acute infectious gastroenteritis in children: Are they effective? Nutrition. 2007;23:498–506.
Pieścik-Lech M, Shamir R, Guarino A, Szajewska H. Review article: The management of acute gastroenteritis in children. Aliment Pharmacol Ther. 2013;37:289–303.
Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, Shamir R, et al. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Pediatr Gastroenterol Nutr. 2008;46:S81–122.
Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut. 1992;33:818–24.
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4.
Thomas DW, Greer FR; American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Section on Gastroenterology. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–31.
Vandenplas Y, De Hert SG, PROBIOTICAL-study group. Randomised clinical trial: The synbiotic food supplement probiotical vs. Placebo for acute gastroenteritis in children. Aliment Pharmacol Ther. 2011;34:862–7.
Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;11: CD003048.
Shamir R, Makhoul IR, Etzioni A, Shehadeh N. Original research: evaluation of a diet containing probiotics and zinc for the treatment of mild diarrheal illness in children younger than one year of age. J Am Coll Nutr. 2005;24:370–5.
Dalgic N, Sancar M, Bayraktar B, Pullu M, Hasim O. Probiotic, zinc and lactose-free formula in children with rotavirus diarrhea: Are they effective? Pediatr Int. 2011;53:677–82.
Sazawal S, Jalla S, Mazumder S, Sinha A, Black RE, Bhan MK. Effect of zinc supplementation on cell mediated immunity and lymphocyte subsets in preschool children. Indian Pediatr. 1997;34:689–97.
Wadhwa N, Natchu UC, Sommerfelt H, Strand TA, Kapoor V, Saini S, et al. ORS containing zinc does not reduce duration or stool volume of acute diarrhea in hospitalized children. J Pediatr Gastroenterol Nutr. 2011;53:161–7.
Passariello A, Terrin G, De Marco G, Cecere G, Ruotolos S, Marino A, et al. Effiacy of a new hypotonic oral rehydration solution containing zinc and prebiotics in the treatment of childhood acute diarrhea: A randomized controlled trial. J Pediatr. 2011;158:e1.
Agustina R, Kok FJ, van de Rest O, Fahmida U, Firmansyah A, Lukito W, et al. Randomized trial of probiotics and calcium on diarrhea and respiratory tract infections in Indonesian children. Pediatrics. 2012;129:1155–64.
Mcfarland LV, Elmer GW, Mcfarland M. Meta-analysis of probiotics for the prevention and treatment of acute pediatric diarrhea. Int J Probiotics Prebiotics. 2006;1:63–76.
Riaz M, Alam S, Malik A, Ali SM. Efficacy and safety of Saccharomyces boulardii in acute childhood diarrhoea: A double blind randomised controlled trial. Indian J Pediatr. 2012;79:478–82.
Narayanappa D. Randomized double blinded controlled trial to evaluate the efficacy and safety of Bifilac in patients with acute viral diarrhea. Indian J Pediatr. 2008;75:709–13.
Alam S. Efficacy of a synbiotic BIFILAC: Questionable study. Indian J Pediatr. 2009;76:660.
Salazar-Lindo E, Miranda- Langschwager P, Campos-Sanchez M, Woo Chea E, Sack RB. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: A randomized, double-blind, placebo controlled clinical trial [ISRCTN67363048]. BMC Pediatr. 2004;4:18.
Tlaskal P, Schramlova J, Kokesova A, Adamus J, Bubakova D, Kocnarova N. Probiotics in the treatment of diarrhoeal disease of children. NAFAS. 2005;3:25–8.
Tlaskal P, Michkova E, Klayarova H, Jerabkova L, Nevoral J, Balackova J. Lactobacillus acidophilus in the treatment of children with gastrointestinal tract illness. Cesk Pediatr. 1995;51:615–9.
Dianawati D, Shah NP. Survival, acid and bile tolerance, and surface hydrophobicity of microencapsulated B. animalis ssp. lactis Bb12 during storage at room temperature. J Food Sci. 2011;76:592–9.
Vanhee LM, Goemé F, Nelis HJ, Coenye T. Quality control of fifteen probiotic products containing Saccharomyces boulardii. J Appl Microbiol. 2010;109:1745–52.
Rerksuppaphol S, Rerksuppaphol L. Lactobacillus acidophilus and bifidobacterium bifidum stored at ambient temperature are effective in the treatment of acute diarrhoea. Ann Trop Paediatr. 2010;30:299–304.
Institut Rosell. M2 ctd Summaries, summary of information contained in the ctd registration file for the product lacidofil capsules. Supporting Information for the registration of the product lacidofil capsules in Indonesia. 2005.
Guarino A, Dupont C, Gorelov AV, Gottrand F, Lee JK, Lin Z, et al. The management of acute diarrhea in children in developed and developing areas: From evidence base to clinical practice. Expert Opin Pharmacother. 2012;13:17–26.
Contributions
BH, IMIW, HG and YV: Designed the study; IMIW, HG: Included the patients; BH and YV: Wrote the manuscript. YV will act as guarantor for this paper.
Conflict of Interest
YV is consultant for Biocodex and United Pharmaceuticals.
Role of Funding Source
Dexa Medica (Jakarta, Indonesia) provided the probiotics and Indofarma (Jakarta, Indonesia) zinc and ORS for free.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hegar, B., Waspada, I.M.I., Gunardi, H. et al. A Double Blind Randomized Trial Showing Probiotics to be Ineffective in Acute Diarrhea in Indonesian Children. Indian J Pediatr 82, 410–414 (2015). https://doi.org/10.1007/s12098-014-1408-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-014-1408-5