Abstract
Type 2 diabetes mellitus (T2DM) is a chronic progressive disease with high morbidity and mortality rates. Previously an adult onset disease, it is now being diagnosed more and more in childhood and adolescence. Lately, Asia has become the epicenter of this epidemic. Childhood T2DM is a new challenge for the pediatrician. Due to similarities in presentation, children may initially be misdiagnosed with Type 1 diabetes mellitus (T1DM). Most oral anti-diabetic agents have not been approved for use in adolescents, and there is a concern for safety of their use. Lifestyle intervention is difficult to conduct, and adherence to recommendations is lower in adolescents than in adults with T2DM. Higher incidence and early onset of co-morbidities, with lack of long term outcomes data make the management problematic. In many communities, due to a shortage of specialists, general practitioners will treat children with T2DM. Guidelines cited in this review are designed to help with the diagnostic process and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Epidemiology
Although still not a common disease in childhood, pediatric type 2 diabetes mellitus (T2DM) is on the rise worldwide. According to the SEARCH study, T2DM accounts for 15–87 % of new diagnosis of pediatric diabetes in the USA, with higher incidence among minority youths (17.0 to 49.4 per 100,000 person-years) compared with 5.6 per 100,000 in non-Hispanic white [1]. Rapid industrialization and “westernization” of nutrition has caused an explosion of obesity and T2DM in Asia as well. In the Western world, T2DM is mostly affecting the minority and socioeconomically disadvantaged youth. In India, T2DM is not a disease of the poor, but rather is seen in urban affluent youth and has an association with obesity. Data from India show a significant increase in diabetes prevalence in both urban (from 13.9 % in 2000 to 18.2 % in 2006) and rural areas (from 6.4 % in 2000 to 9.2 % in 2006) [2]. Importantly, the proportion of young to middle-aged individuals with T2DM is higher in developing countries [3]. According to the International Society for Pediatric and Adolescent Diabetes (ISPAD), T2DM makes up nearly 60 % of new pediatric cases in Japan, 37 % in Hong Kong, and 50 % in Taiwan. In Japan however, ~30 % of youth with T2DM are not obese, and in Asian-Indian urban children, half of those with T2DM have normal weight, which is different from UK and USA [4, 5].
Diagnosis and Differential Diagnosis
Regardless of age and type, diagnosis of diabetes is based on the following criteria, which are accepted worldwide:
-
Symptoms of diabetes plus casual plasma glucose concentration ≥ 11.1 mmol/L (200 mg/dL).
or
-
Fasting plasma glucose ≥ 7.0 mmol/L (≥ 126 mg/dL) (no caloric intake for at least 8 h).
or
-
2 h postload glucose ≥ 11.1 mmol/L (≥ 200 mg/dL) during an oral glucose tolerance test (OGTT).
(The test should be performed using a glucose load of 1.75 g/kg of body weight to a maximum of 75 g)
or
-
HbA1c ≥ 6.5 % *
*Consider variation of assay used and individual variation of the relationship between blood glucose and HbA1c
It is not easy to differentiate T2DM from T1DM in children. With the epidemic of obesity, the typical phenotypical features of diabetes are becoming blurred, making it more difficult to distinguish between the two. T2DM is characterized by insulin resistance with beta cell defect and progressive decline of insulin secretion, while T1DM is caused by insulin deficiency which is mostly due to autoimmune islet cell destruction. In youth however, the presence or absence of diabetes antibodies cannot be used as the “gold standard.” Various studies show relatively high prevalence of positive diabetes autoantibodies in adolescents with typical T2DM phenotypes and course. The SEARCH study reported that 21 % of children between ages 10 and 19 y diagnosed with T2DM were positive for GAD-65 antibody, but TODAY study found antibodies in 9.8 % of its cohort [1, 6]. When antibody-positive children with clinical T2DM were compared to those who were antibody-negative, the youth with antibodies had lower body mass index and progressed towards insulin dependency more rapidly [6]. In populations with high incidence of T2DM, antibody testing for diagnostic purposes may not be necessary, but knowledge of autoimmunity can guide the therapy. C-peptide level in adolescents varies greatly at the time of presentation, depending on islet cell function. In complex cases only the clinical course of disease and response to insulin-sensitizing agents over a period of time can determine diagnosis.
Monogenic diabetes includes neonatal diabetes, subtypes of maturity onset diabetes of the young (MODY), and mitochondrial diabetes and is characterized by lack of diabetes autoimmunity. Some types respond to sulfonylureas. Genetic testing for types of MODY, and neonatal diabetes are commercially available.
Other potential causes of diabetes and hyperglycemia must be considered, such as exocrine pancreas dysfunction from cystic fibrosis, endocrine abnormalities (Cushing syndrome, pheochromocytoma), or polycystic ovarian disease (PCOS) and drug-induced (glucocorticoids, anti-retroviral drugs, or chemotherapeutic agents).
Clinic Presentation
Onset of T2DM is typically insidious. A third of youth is identified by routine laboratory screening or during a non-related illness. Common signs and symptoms include polydipsia, polyuria, and polyphagia, often without weight loss. Although obese children can develop autoimmune diabetes as well, patients with T2DM with negative antibodies have more pronounced signs of insulin resistance (acanthosis nigricans), greater BMI, higher fasting C-peptide levels, lower HbA1c values, and higher lipid levels at the presentation [6]. A recent publication by the TODAY Group has shown that in a large cohort of youth, T2DM often presented with other metabolic abnormalities: dyslipidemias (80.5 %), hypertension (13.6 %), and microalbuminuria (13 %). Dyslipidemias were most commonly represented by low high-density lipoproteins (HDL), and elevated triglycerides (TG) [7]. Symptoms can include fatigue, slow healing infections/wounds, blurry vision, and skin changes. Recurrent yeast infections (vaginitis) are common in girls. Table 1 summarizes the clinical characteristics of type 1 and 2 diabetes mellitus in youth.
More severe presentations include diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state (HHS). Ketosis is caused by long standing toxic effect of hyperglycemia (glucotoxicity), which adversely affects insulin secretion, and causes a relative insulinopenia. Prevalence of DKA in youth with T2DM can be as high as 13 %, while rare in adults, and is more common in Hispanic and African–American adolescents. The same study also found that patients with T2DM had more severe hyperglycemia and required longer insulin infusion therapy and had a longer hospital stay for the same severity of acidosis compared to those with T1DM [8]. Diagnostic criteria of HHS include: serum glucose > 33 mmol/L (600 mg/dL), serum osmolality > 330 mosm/L, mild ketosis and lack of acidosis. There are just a few reports on incidence of HHS in children and youth, indicating high mortality rate [9].
Treatment
There are many challenges in treating T2DM in children and youth, including lack of access to care, socioeconomic limitations, and cultural beliefs preventing families from seeking medical help. Treatment of T2DM targets insulin resistance and relative insulinopenia, but most of the current treatment paradigms are based on adult data, and may not always apply to children. Detailed treatment regimens are beyond the scope of this review article, but general treatment options are as follows.
Lifestyle Intervention
Dietary recommendations aim at weight loss in children. The following steps have proven to be effective in obese youth: (1) eating regular meals and snacks; (2) reducing portion sizes; (3) choosing calorie-free beverages, except for milk; (4) limiting juice to 1 cup; (5) increasing consumption of fruits and vegetables; (6) consuming 3 or 4 servings of low-fat dairy products per day; (7) limiting intake of high-fat foods; (8) limiting frequency and size of snacks; and (9) reducing calories consumed in fast-food meals [10]. It is not recommended to restrict caloric intake to less than 1,200 kcal/d and prescribe difficult-to-adhere diets such as ketogenic and protein-sparing, unless patients are closely monitored by a specialist. Sugar and sugar-containing products should be limited.
Regular physical activity should be initiated and “screen time” should be limited to 2 h a day. Maintaining moderate-to-vigorous exercise for at least 60 min daily can be difficult particularly with no resources or appropriate family support. A written recommendation or plan as well as sensitivity and acknowledgement of family’s circumstances can improve adherence and outcomes.
Pharmacological Interventions
Biguanides (metformin) are the most commonly used insulin sensitizing agents, and the only oral agents approved by the USA Food and Drug Administration (FDA) for use in children. Metformin acts primarily on the liver by suppressing gluconeogenesis and improves glucose uptake by muscles. Metformin, used as monotherapy can lower HbA1c by 0.5–1 % [11]. Metformin is usually dosed for children between ages 10 and 16 y; initial starting dose is 500 mg orally twice a day with increases made weekly as needed by 500 mg/d in divided doses to a maximum daily dose of 2,000 mg/d. Metformin needs to be taken with meals and caution is advised for those taking medications that affect renal function. Although hypoglycemia due to intake of metformin is rare, caution is also advised for those who have insufficient caloric intake, participate in strenuous activity, or those taking other hypoglycemic medications. Lactic acidosis is the most serious side effect, but this has not been reported in children. Suggested monitoring while on metformin includes fasting blood glucose, HbA1c, initial and periodic monitoring of hemoglobin, hematocrit, and red blood cell indices; and renal function (baseline and annually).
Thiazolidinediones or TZDs (rosiglitazone, pioglitazone) are peroxisome proliferator-activated receptor (PPAR-γ) agonists. They promote glucose uptake by skeletal muscles, liver and adipocytes. TZDs are not approved for use in children however, rosiglitazone has been found safe and effective within a clinical trial in 10–18 y old subjects [12]. Hepatotoxicity, fluid retention, and weight gain are concerns, and monitoring of liver enzymes is recommended. TZDs can be used as a single agent, with metformin, and with insulin. Hypoglycemia is a common side effect, and caution is needed when used with insulin.
Sulfonylureas (insulin secretagogues) have limited use in pediatric T2DM. Children diagnosed at a young age have limited islet cell reserve, and overstimulation may accelerate beta cell demise and insulin dependency. Major side effects are hypoglycemia and weight gain. Short-acting or prandial sulfonylureas (meglitinide analogs) can improve postprandial control, but have same side effects, and require 3–4 times a day dosing which is difficult for most adolescents.
Glucosidase inhibitors (acarbose) prevent glucose absorption in small intestine, and have mild hypoglycemic effect. Side effects are flatulence and diarrhea.
Incretin-mimetics Two new classes have been used with success in adults: glucagon-like-peptide-1 receptor agonists and dipeptyl peptidase-4 inhibitors. These drugs increase insulin secretion proportionate to blood glucose (BG) concentration, suppress glucagon, prolong gastric emptying, and promote satiety, and aid to weight loss. There are ongoing trials to evaluate safety and efficacy in adolescents.
Insulin is commonly used in youth with T2DM. It is the most potent hypoglycemic agent. Newer “designer” preparations allow for various combinations to be created and to tailor therapy to individual needs. However, many children fear needles, which can compromise adherence. Major side effects are hypoglycemia and weight gain.
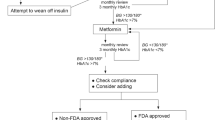
In 2013, The American Academy of Pediatrics published clinical practice guideline based on modalities that have been shown to affect clinical outcomes in pediatric population [13]. At the onset of T2DM, or for a known diabetes patient, who presents with ketosis, (DKA or HHS) insulin therapy is indicated, and children should be treated with intravenous fluids by a specialist. Insulin is also recommended for a new onset T2DM with a random serum glucose > 250 mg/dL and HbA1c > 9 %. Insulin can rapidly restore glycemic control, reduce glucotoxicity, improve insulin sensitivity, and recovery of islet cell function. Metformin can be later added, and insulin gradually reduced and stopped. Initial insulin therapy will also allow for adequate time for differentiating T1DM from T2DM in ambiguous cases. If weaning from insulin is not successful, one may consider wrong diagnosis or non-adherence with prescribed regimen. It is important to provide patients with appropriate technical skills, since insulin may be required again later in the course of disease. In youth presenting with mild hyperglycemia, metformin along with lifestyle change is now recommended as the first-line therapy since in adolescents metformin is more effective than lifestyle change alone. Metformin is usually well tolerated and safe. Gastrointestinal side effects can be avoided by gradually increasing the dose from 500 mg a day to 2,000 mg a day. Metformin facilitates weight loss or weight maintenance, and has a low risk for hypoglycemia. Most adolescents would choose an oral medication over an injection, but the adherence with pills in many cases is less than optimal.
Recently, a large multi-center trial (TODAY) revealed that metformin alone was inadequate in the majority of youths, and addition of rosiglitazone to metformin is superior to metformin alone in preserving glycemic control [12]. Routine use of rosiglitazone however cannot be recommended due to a lack of FDA approval and emerging evidence of potential side effects. The same trial demonstrated failure of oral diabetes therapy and insulin dependency in 45.6 % of subjects in an average of 3.86 y, which confirms earlier reports on rapid progression of disease in youth [13].
Therapy adjustments are based on HbA1c. Most practices aim at maintaining HbA1c at < 6.5–7 %, and monitoring HbA1c every 3 mo. A decision tree included in ISPAD Clinical Practice Consensus Guidelines can provide a practitioner with guidance [4]. Routine blood glucose testing is recommended 2–4 times a day, based on whether diabetes is in good control, and if the patient takes insulin, or is at risk for hypoglycemia.
Treatment of Co-Morbidities
A large percentage of adolescents with T2DM have one or more co-morbidities present at the time of diagnosis. ISPAD recommends urine microalbumin testing and examination for retinopathy at diagnosis and annually thereafter. Blood pressure should be monitored at every visit. Testing for dyslipidemia should be performed soon after diagnosis when glucose control is established, and annually. Additionally, evaluation for non-alcoholic fatty liver disease (NAFLD), pubertal, menstrual irregularities and obstructive sleep apnea should be done at diagnosis and addressed as indicated. Detailed information is available in the ISPAD guidelines on complications [5].
Confirmed hypertension (BP >95 % for age, gender, and height) or albuminuria should be treated with an angiotensin converting enzyme (ACE) inhibitor or, if not tolerated, an angiotensin receptor blocker. Combination therapy may be required if hypertension or albuminuria does not normalize with single agent.
Cholesterol goal is LDL-C <2.6 mmol/L (100 mg/dL). If LDL is borderline (2.6–3.4 mmol/L; 100–129 mg/dL) or elevated (3.4 mmol/L; 130 mg/dL), repeat lipid profile should be performed in 6 mo and dietary intervention to decrease total and saturated fat initiated. If there is no improvement after 3–6 mo with diabetes control and diet, pharmacotherapy is warranted. Statins have been shown to be safe and effective in children as in adults and should be the first pharmacologic intervention, although long-term safety is not known.
Prevention and Screening
Currently, there is no justification for screening all youth for T2DM. In high-risk populations, and in children at risk, targeted screening using fasting serum glucose, or 2-h post glucose challenge may be beneficial, and help to identify youth with impaired glucose tolerance or “pre-diabetes”. ISPAD defines children at risk for T2DM as follows:
-
Children with BMI 85-95th percentile.
-
If there is an immediate family history of type 2 diabetes, early cardiovascular disease (CVD), or if there are signs of insulin resistance (acanthosis nigricans, dyslipidemia, hypertension, PCOS)
-
Asian children regardless of BMI, if history of abnormally low or high birth weight, or family history of diabetes.
-
Children with BMI > 95th percentile, regardless of family history or associated features.
Prevention of Type 2 Diabetes
There is no question that genetic factors determine a child’s risk for T2DM, however, obesity is the leading environmental factor, and accelerates manifestations and progression of disease. Relatively minimal weight loss can decrease the rate of diabetes in at risk populations [14]. Unless obesity is addressed, societies will see further global increase in incidence and prevalence of T2DM in children. Prevention of obesity should target eating patterns at home, and in school. Health education should be provided by schools and health care professionals. Communities and governments are charged with providing access to healthy foods and opportunities for physical activity. Major investment in infrastructure may be needed to promote healthier lifestyles.
Conclusions
T2DM is emerging at a much younger age, with more pronounced islet cell defect and rapid progression toward insulin dependency. There are significant barriers to care since the disease disproportionately affects minorities and the underprivileged. Most recent recommendations suffer from lack of long-term data. Primary care physicians find themselves on the frontlines without much training or experience in pediatric T2DM. Seeking for advice of a specialist, and staying up to date in this rapidly evolving area is crucial for proper diagnosis and treatment of this challenging disease.
References
Writing Group for the SEARCH for Diabetes in Youth Study Group, Dabelea D, Bell RA, D’Agostino RB Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–24.
Ramachandran A, Mary S, Yamuna A, Murugesan N, Snehalatha C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care. 2008;1:893–8.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Type 2 diabetes in Asian–Indian urban children. Diabetes Care. 2003;24:1022–5.
International Diabetes Federation. The Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence. 2011.
Klingensmith GJ, Pyle L, Arslanian S, Copeland KC, Cuttler L, Kaufman F, et al; TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype. Diabetes Care. 2010;33:1970–5.
Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: The TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–67.
Sapru A, Gitelman SE, Bhatia S, Dubin RF, Newman TB, Flori H. Prevalence and characteristics of type 2 diabetes mellitus in 9–18 year-old children with diabetic ketoacidosis. J Pediatr Endocrinol Metab. 2005;18:865–72.
Pinhas-Hamiel O, Zeitler P. Clinical presentation and treatment of type 2 diabetes in children. Pediatr Diabetes. 2007;8:16–27.
Loghmani ES. Nutrition therapy for overweight children and adolescents with type 2 diabetes. Curr Diab Rep. 2005;5:385–90.
Jones KL, Arslanian S, Peterkova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: A randomized controlled trial. Diabetes Care. 2002;25:89–94.
TODAY Study Group, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–56.
Levitt Katz LE, Magge SN, Hernandez ML, Murphy KM, McKnight HM, Lipman T. Glycemic control in youth with type 2 diabetes declines as early as two years after diagnosis. J Pediatr. 2011;158:106–11.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Conflict of Interest
None.
Role of Funding Source
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tieh, P., Dreimane, D. Type 2 Diabetes Mellitus in Children and Adolescents. Indian J Pediatr 81, 165–169 (2014). https://doi.org/10.1007/s12098-013-1193-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-013-1193-6