Abstract
Objective
To estimate the prevalence of the Inborn Errors of Metabolism (IEM), evaluate biomarker distributions and determine benefits of screening for the inborn errors of metabolism in Andhra Pradesh, India, using Tandem Mass Spectrometry (MS/MS).
Methods
The 4,946 newborns born during the period 2006–2008 in four major Government Maternity Hospitals in a rural district in Andhra Pradesh, India, were screened at an established newborn screening laboratory in the US using their previously established norms.
Results
Forty-seven neonates had out-of-range results (5 high probability; 28 low probability; 14 indeterminate). Two infants with disorders (carnitine uptake disorder and isovaleric aciduria) identified by screening are currently doing well. One infant with presumed glutaric aciduria type II, was deceased at the time of reporting. Another infant, with glutaric aciduria type I, became symptomatic and died at the age of 1 year despite early detection and treatment. A comparison of the concentrations of biomarkers among babies born in India and those born in Massachusetts, US, was also undertaken and significant differences were noted.
Conclusions
A high prevalence of disorders was observed, but to estimate the true extent of the IEM in India larger studies are required. This study also illustrates challenges encountered in disease management highlighting the importance of considering the access to confirmatory testing and continuing clinical care before implementing any large-scale NBS for conditions with resource-intensive health needs such as the IEM detected by MS/MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Newborn screening provides an opportunity for identification of, and consequently early intervention in, pre-symptomatic infants with serious inborn disorders to prevent or mitigate adverse outcomes associated with these conditions. Screening is accomplished, primarily, by analysis of biomarkers in a micro-sample of blood collected from newborns using a spectrum of analytic methodologies. Currently, it is feasible to screen for more than sixty conditions in various categories (Endocrine, Inborn Errors of Metabolism, Hemoglobinopathies, Infectious and Immunological) and this number is only expected to increase over time with advancements in technology [1, 2]. The panel of disorders screened for, and the cohort of newborns screened, varies substantially in the different parts of the world [3]. In many nations newborn screening is performed as standard practice on all babies born, while in others newborn screening is not feasible, or is available to only a subset of neonates.
Although efforts are underway to implement universal newborn screening, for at least a few common disorders, at present newborn screening is not performed on all newborns in India [4, 5]. Previous pilot studies conducted in India have shown a high prevalence of treatable disorders [6]. Chromatographic (TLC and HPLC), electrophoretic (cellulose acetate and agarose) and ELISA based assays were employed in the screening process and thus the metabolic disorders detected were limited. One of the more recent technologies introduced into newborn screening is tandem mass spectrometry (MS/MS). MS/MS allows simultaneous, rapid and accurate detection of multiple amino acids and acylcarnitines, and hence has expanded the spectrum of metabolic disorders identifiable by screening [7–10]. The authors report their experience of the first pilot study conducted in India to screen for inborn errors of metabolism using this methodology.
Material and Methods
Cohort
The 4,946 newborns born during the period 2006–2008 in four major Government Maternity Hospitals in a rural district in Andhra Pradesh, India, were screened. Heel prick capillary blood collected on S&S 903 filter paper was transported to the New England Newborn Screening Program (NENSP) via courier. The screening laboratory checked the appropriateness of samples for analysis. Unsatisfactory specimens were rejected and re-sampling was done for those rejected. The majority of technically satisfactory samples (60%) were obtained between day of life (DOL) 1–3 and while the newborns were still in the hospital. The remaining specimens (40%) were collected when the neonates presented to the hospital clinic for a routine check. Consent for inclusion in the study was obtained in accordance with the guidelines provided by the Indian council of Medical Research.
Analysis
The laboratory procedures, as previously reported [11], involved extraction of biomarkers from the dried blood spots into a methanol solution with stable isotope-labeled internal standards, and derivatization for analysis by MS/MS. Table 1 shows the disorders screened for and the primary markers measured for screening. The laboratory’s previously established cutoffs, based on expected ranges in babies born in New England, were utilized and no separate cut-offs were established for specimens from babies in India. Specimens with biomarker values that were above New England Newborn Screening Program (NENSP) cut-offs were retested.
Reporting and Follow-Up of Out-of-range Results
All out-of-range results were sorted into one of the three distinct categories: “Indeterminate”, “Low Probability” and “High Probability” as per protocols in use by NENSP [Sahai I, Eaton RB. Customized “FACT SHEETS”: Category-Based Information Provided When Reporting “Positive” Results. Poster. 2008 Newborn Screening and Genetic Testing Symposium, San Antonio, TX. 3–6 November 2008].
The criteria for assigning “positive” results into one of these categories were developed after a retrospective analysis of archived results from babies screened by NENSP. The biochemical parameters used in defining the criteria include both magnitude of the deviation of the primary marker from the population mean and the biochemical profiles. To quantify biochemical profiles, NENSP uses “Discriminator Indices” that essentially are mathematical combinations of several characteristic analytes [Sahai I, Zytkovicz T, Grady T, et al. Improving Interpretation of “Positive” Newborn Screening Results: A metabolomic approach for analysis of MS/MS data in developing “Predictive Indices”. Poster 2007 Newborn Screening and Genetic Testing Symposium, Minneapolis, MN. 7–10 May 2007].
Results were communicated to the Principal Investigator (PI) in India along with the subcategory-based recommendations. For High Probability results, an expedient evaluation and confirmatory work-up was recommended.
For the “Low Probability” results, additional intervention was suggested, but it varied based on the analyte involved. For markers whose deviations were likely to increase in affected neonates, but are associated with disorders that could present critically within days of birth (e.g. Cit & ASA), an immediate clinical evaluation and some testing (e.g. ammonia) was recommended by the PI. However confirmatory testing was recommended only if a repeat specimen also tested abnormal. For markers that may decrease over time even among affected neonates (e.g. C8, C5-DC & C14: 1), confirmatory testing was recommended in addition to a repeat screen.
For “Indeterminate” results, a repeat filter paper screen was requested and confirmatory tests suggested only if the repeat specimen also tested abnormal. Evaluation and confirmatory testing was performed at the metabolic clinic in the DNA Diagnostic and Fingerprinting Laboratory in Hyderabad.
Results
Demographic Data
Of the 4,946 initial specimens received, 4,870 specimens (Males 2,620; Females 2,238; No gender data on 12) were technically satisfactory for testing. The distribution of age when specimens were collected is shown in Fig. 1; approximately 60% of the samples were collected within 24–72 h of birth. In contrast, of the 134,539 initial technically satisfactory specimens received from babies born in Massachusetts (MA) in the same period, 94% were collected within 24–72 h of birth. Also notable in this study was that only 2% of specimens from India were from babies weighing less than 1.5 kg or in the NICU. In comparison 7% of babies born in MA weighed less than 1.5 kg or were in the NICU when the initial specimen was collected.
Distribution of Markers
Table 2 shows the means, median, 1st and 99th percentile of the distribution of the primary biomarkers analysed by MS/MS in neonates born in India. Also shown for comparison are the parameters of the same biomarkers in the babies born in MA. In view of the significant differences in the age at specimen collection and in the number of VLBW/NICU babies in the two populations, only specimens collected between 24–72 h from non-NICU babies weighing more than 1.5 kg were included for the comparison. Comparison was also restricted to specimens tested on the same tandem mass spectrometer (n = 29,413 from MA and n = 2,852 from India). Comparison of the concentrations of biomarkers in the two populations using T-test analysis was performed and p-values are shown in the last column. Statistically significant differences were noted in the concentrations of most biomarkers, with the amino acids (except Leucine) and acylcarnitines (except free carnitine, C16 and C18:1) being higher in the babies born in Massachusetts as compared to babies born in India.
Out-of range Results
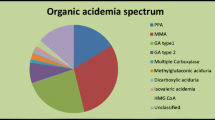
Of the 4,896 babies whose screening specimen was collected within 30 days of life, 47 had out-of-range results based on previously established NENSP cutoffs. The marker abnormality, category breakdown and final outcomes are shown in Table 3.
Confirmatory Testing
Plasma amino acids and urinary organic acid analyses were performed on neonates with out-of-range markers, where required, at the metabolic clinic of the DNA Diagnostic and Fingerprinting Center, Hyderabad. However, in view of the difficulty and expense involved in getting certain confirmatory studies such as plasma acylcarnitine analysis on babies included in the study, these studies were not performed. Instead, the authors relied on acylcarnitines measured on repeat dried blood spots for decision making and monitoring. In most cases molecular analyses were not undertaken.
Clinical History and Outcomes of Cases
Case I-Carnitine Uptake Deficiency (CUD)
This male baby was the first-born child of a consanguineous marriage (parents were first cousins). He was noted to have low free carnitine (C0), and other markers in a profile consistent with CUD on newborn screening. At his initial clinical evaluation on DOL 8, he was clinically asymptomatic and his EKG was normal. Repeat testing revealed persistence of low free carnitine and he was started on oral carnitine 100 mg/kg body weight. At 6-months of age, carnitine supplementation was discontinued, but this resulted in a drop in his free carnitine to significantly low levels (3.6 μM). Carnitine supplementation was restarted, and carnitine level has remained in the normal range since. He is being followed periodically and at his last evaluation the developmental milestones were normal and an EKG was normal. A second child was born to his parents last year, and does not appear to be affected based on a normal newborn screen.
Case II- Glutaric Aciduria Type II (GA-II)
This female child was born to a consanguineous couple (parents were first cousins). Significant elevations of multiple acylcarnitines (C4, C8, C5DC, C14), in a pattern consistent with GA-II, were noted in the screening specimen collected on DOL 3. Although the family was contacted immediately after the results became available, the baby was already deceased at the time. According to the parents the neonate had died suddenly on day of life 4. No confirmatory testing could be performed, however family was provided genetic counseling based on the presumptive diagnosis of GAII.
Case III-Glutaric Aciduria-I (GAI)
This male neonate was the first-born child of non-consanguineous parents. He was born at 36-wks gestation with a birth weight of 1.7 kg. His newborn screening revealed an elevation of C5DC in a pattern consistent with GAI. At his initial evaluation he was asymptomatic, active and feeding well. Urinary organic acid analysis revealed elevated glutaric acid and 3-hydroxyglutaric acid, confirming the diagnosis of GAI. In addition to the above metabolities, increased excretion of 2-ketoglutaric acid and succinic acid was noted. Treatment with carnitine and riboflavin and a low protein diet, with special emphasis on avoidance of foods high in lysine and tryptophan, was initiated. Since the specialized medical formula to provide the recommended daily allowances for the essential amino acids was not available at an affordable cost, the family opted not to continue with this. Over the course of time the infant developed developmental delay with myoclonic seizures and failure to thrive, and expired at 1 year of age.
Case IV-Isovaleric Aciduria (IVA)
One baby in the cohort was the third child of a couple whose two previous children had died of metabolic acidosis. This male baby was born at 34-wks weighing 1000 g. In view of the low birth weight and family history, he was managed in the NICU and was started on a low protein formula on DOL 3. The screening specimen, collected on DOL 2, revealed a significant elevation of C5 in a pattern consistent with IVA. Urinary organic acid analysis using GC-MS confirmed the diagnosis of IVA. His neonatal period was notable for a few episodes of diarrhea and severe anemia requiring blood transfusion. He has remained on a low protein diet and carnitine, and currently at the age of 3-years, his cognitive and motor development is normal.
Discussion
On comparing the demographic data of all the babies screened from India with those of babies born in Massachusetts, significant differences were noted in the percentage of babies who were noted to be in Intensive/Special Care Units (ICU) or had birth weights under 1.5 kg (2% in India; 7% in MA), and in the age at specimen collection. The authors, therefore, restricted each population to specimens collected at 24–72 h of age from non-NICU babies weighing over 1.5 kg, and analyzed on the same instrument, for further comparisons. It is interesting to note that even with these restrictions the concentrations of the biomarkers differed significantly among the normal babies born in India as compared to the babies born in Massachusetts. A similar difference was not noted among babies born in Massachusetts when compared to babies born in other New England states also tested by NENSP. In order to explain the differences, the authors compared other factors that may have contributed to the differences. Table 4 shows the demographic data of the two populations (after restriction to non-NICU babies of similar age and weight as described previously). One notable difference was the percentage of babies on breast milk (99% in India; 66% in MA). The authors feel that variations in norms related to feeding and maternal diet, possibly in addition to some founder effects affecting the metabolic pathways, are responsible for the observed differences in marker concentrations. The observed variations in biomarker concentrations among the populations raise the question for a need for population-specific screening cut-offs, but larger studies would be required to confirm that this is needed or justified to distinguish affected babies from normals. Careful analysis of the profiles using ratios and indices may be able to compensate for such differences.
The spread of all positive screens into the three categories was substantially different among babies from India with those from MA. Of the 48 positive screens among babies born in India, 30% were in the “Indeterminate Category” and 60% were in the “Low Probability Category”. On the other hand, the category-based distribution pattern was reversed among babies born in MA, with 60% of screens falling in the “Indeterminate Category” and 30% in the “Low Probability Category”.
This difference was primarily due to the number of babies with an elevation of multiple markers, with at least 3 among LEU, C5, C8, C12, C14:1, C14 and C16 above their individual cut-offs, and the remaining in the high range of normal. Of the 4, 896 initial specimens from India 14 were noted to have this profile, while of more than 100,000 specimens from MA tested during the same period only 2 were noted to have this pattern. An elevation of several markers in the pattern above is seen in neonates with Glutaric Aciduria-II; however all cases of GA-II detected previously by NENSP have had much higher concentrations (and fell in the “High Probability” category). When a much larger cohort of neonates from MA was analyzed previously, those babies with a similar elevation of multiple markers in the “Low Probability” category, and who had extensive diagnostic testing were all concluded to be false-positives; in these cases a maternal riboflavin deficiency or a transient mitochondrial dysfunction was suggested. The elevations in the 14 babies from India in the study resolved on retesting but an extensive diagnostic work-up was not performed. Although it is possible that some of the 14 neonates with this profile had GA-II, it is unlikely. Notably several markers (Leucine, C5 and C16) in this pattern of multi marker elevation have higher overall mean/median concentrations in specimens from babies from India. This difference in the median concentrations is likely related to the large number of neonates with the above-mentioned specific pattern of out-of-range results, but the cause remains unclear. A higher prevalence of maternal riboflavin deficiency in the Indian population is a possibility but data were not available to confirm this speculation.
To the best of authors’ knowledge, this was the first unselected population-based newborn screening study conducted for disorders using MS/MS in a region in India. More recently, the state of Goa has begun to screen all newborns born in a Goa Government healthcare facility for several conditions. The authors observed a high prevalence of inborn errors of metabolism to the extent of one in every two thousand newborns in the region. This pilot underscores the necessity of performing larger population-based newborn screening studies to estimate the true extent of the inborn errors of metabolism in India. It also illustrates some challenges that are likely to be encountered when screening for these disorders is introduced into the general population. The authors experienced significant difficulties in obtaining some confirmatory studies for infants who screened positive, due to lack of accessibility to many of the families to diagnostic-quality laboratory analyses. Procuring the specialized diets, the mainstay of treatment, for individuals confirmed to have a disorder was another arduous task because these rare formulas are not manufactured in India and have to be imported from outside the country at high expense. Benefits of screening can only be realized if infants identified by screening receive the necessary follow-up intervention and life-long therapy [12–14]. Hence it will be imperative to consider the availability of, and access to, confirmatory testing and continuing clinical management before implementing any large-scale population-based newborn screening for conditions with resource-intensive health needs, such as the inborn errors of metabolism detected using MS/MS.
References
Wilcken B. Recent advances in newborn screening. J Inherit Metab Dis. 2007;30:129–33.
Sahai I, Marsden D. Newborn Screening. Crit Rev in Cli Lab Sci. 2009;46:55–82.
Pollitt RJ. International perspectives on newborn screening. J Inherit Metab Dis. 2006;29:390–6.
Miller FA. The complex promise of newborn screening. Indian J Med Ethics. 2009;6:142–8.
Kapoor S, Kabra M. Newborn screening in India current perspectives. Indian Pediatr. 2010;47:219–23.
Rama Devi AR, Naushad SM. Newborn screening in India. Indian J Pediatr. 2004;71:157–60.
Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–4.
Chace D, Naylor E. Expansion of newborn screening programs using automated tandem mass spectrometry. Ment Retard Dev Disabil Res Rev. 1999;5:150–4.
Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–817.
Rinaldo P, Tortorelli S, Matern D. Recent developments and new applications of tandem mass spectrometry in newborn screening. Curr Opin Pediatr. 2004;16:427–33.
Zytkovicz TH, Fitzgerald EF, Marsden D, et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin Chem. 2001;47:1945–55.
Wilcken B. Mini-symposium: newborn screening for inborn errors of metabolism—clinical effectiveness. Inherit Metab Dis. 2006;29:366–9.
Padilla C, Krotoski D, Therrell Jr B. Newborn screening progress in developing countries-overcoming internal barriers. Semin Perinatol. 2010;34:145–55.
Wilcken B. Improving child health—newborn screening for all? Ann Acad Med Singapore. 2008;37:3.
Acknowledgements
The authors are grateful to the CDFD Director for authorizing the study and the Department of Biotechnology, Government of India, for financial support.
Conflict of Interest
None.
Role of Funding Source
Department of Biotechnology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahai, I., Zytkowicz, T., Rao Kotthuri, S. et al. Neonatal Screening for Inborn Errors of Metabolism Using Tandem Mass Spectrometry: Experience of the Pilot Study in Andhra Pradesh, India. Indian J Pediatr 78, 953–960 (2011). https://doi.org/10.1007/s12098-011-0398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-011-0398-9