Abstract
Objective
To measure mesenteric fat thickness with ultrasound scan in neonates and to assess the correlation with waist circumference.
Methods
Ninety five healthy newborns had the maximum thickness of mesenteric leaves measured by ultrasound examinations of abdomen with an Envisor scanner (Philips Ultrasound, Bothell, Wash) and a L12-5 transducer (Philips Ultrasound). The correlation between the thickness of mesenteric leaves with abdominal waist was calculated.
Results
Maximum thickness of mesenteric leaves ranged from 0.24 to 1.00 mm \( ({\hbox{x}} = 0.{57}\pm 0.{17}) \). There was a significant negative correlation between abdominal waist (AW) and mesenteric fat thickness (r = −0.384; p < 0.001).
Conclusions
Mesenteric fat thickness in newborns is inversely associated with waist circumference. Higher visceral adiposity in neonates may be a protective mechanism from intrauterine growth restriction however this could persist into adulthood life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The obesity epidemic is a public health concern in high income countries and is now dramatically on the rise in low- and middle-income countries [1]. Obesity is a major risk factor for cardiovascular diseases and a number of others chronic diseases [1, 2]. Abdominal fat is a predictor of risk for obesity-related diseases and it is well established that a more central fat distribution is associated with increased risk of cardiovascular disease [2, 3]. Recently many studies have demonstrated that adipose tissue is metabolically active and is a source of a number of hormones, cytokines and vasoactive peptides that have direct effects on the vasculature to increase cardiovascular risk [4, 5]. Visceral adipose tissue, particularly mesenteric fat, is metabolically more active than subcutaneous fat [2]. Mesenteric fat is more sensitive to the lipolytic effects of catecholamines to release more free fatty acid (FFA) which leads to an increase of gluconeogenesis, impairment of metabolism and action of insulin and increased lipoprotein synthesis [6].
Waist circumference (WC) has been widely used to measure the degree of central fat distribution and it is more closely linked to cardiovascular disease risk factors than is body mass index (BMI) [2]. However, WC can not distinguish subcutaneous fat accumulation in the abdominal region from visceral fat accumulation especially mesenteric fat. Mesenteric fat can be recognized by ultrasound scan and it is the only imaging method which can visualize individual mesenteric leaves [7]. Ultrasound scan is a noninvasive and low cost method which can be used to image body fat without radiation exposure and have a high correlation with radiographic computed tomography. This method has been used in adults [8, 9] and to our best knowledge there are only a few studies in children and none in infants or neonates [10, 11].
Some epidemiological studies pointed out that fat distribution could be programmed in fetal life and that adaptation to a limited supply of intrauterine nutrient might increase the risk of non-transmissible chronic diseases in later life [12, 13]. Visceral adiposity and hyperinsulinemic insulin resistance which leads to type 2 diabetes or metabolic syndrome starts with intrauterine growth restraint (IUGR) [14]. An adverse nutritional environment in utero causes defects in the development of body organs leading to a ‘programmed’ susceptibility that interacts later with diet and environmental stresses to cause overt disease many decades later [15]. The “thrifty phenotype hypothesis” establishes that a predisposition to adult obesity is a consequence of an adaptation to malnutrition by the developing fetus [16]. However, the distribution of visceral fat at birth is unknown. We hypothesize that fetal growth restraints may increase mesenteric fat at birth like a mechanism to offer more chances to survival in a limited supply of intrauterine nutrient. The aim of our study is to measure mesenteric adiposity with ultrasound scan in a sample of newborns and to assess the correlation with WC.
Material and Methods
Ninety-five healthy newborns (50 boys and 45 girls) were recruited from the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), Northeast of Brazil. All had Apgar score ≥7 and gestational age ≥37 weeks. The study was approved by the Research Ethics Committee of IMIP and all the mothers gave informed written consent.
All newborns underwent ultrasound examinations of abdomen with an Envisor scanner (Philips Ultrasound, Bothell, Wash) and a L12-5 transducer (Philips Ultrasound). A complete survey examination was performed with special attention to identify the mesenteric leaves, which represented as elongated structures with highly reflecting peritoneal surfaces. Small vascular structures could be seen within it. The mesenteric leaves were divided from each other by specular echoes corresponding to their peritoneal surfaces. The maximum thickness was measured (Fig. 1). Many measurements were made on each ultrasound examination, and the thickest mesenteric leave was used for the analysis. All measurements were carried out by a single skilled operator (E.J.), with the neonates lying in supine position and without compression of the abdominal wall with the transducer. The linear-array probe was kept perpendicular to the skin, on the upper median abdomen and longitudinal scanning was done under the xiphoid process.
All neonates were weighed and had the height measured within the first 24 h of life with a digital scale to the nearest 0.1 Kg and a portable standiometer of 1 cm, respectively. Using a plastic measuring tape, waist circumference was measured midway between the lower rib margin and the iliac crest. Body mass index (BMI) was calculated (Kg/cm2).
In a subgroup of ten neonates with weight, length and BMI comparable to the whole group, the measurements of mesenteric fat thickness were repeated three times and the obtained coefficient of variation was 6.54% (range 4.45–7.58%).
Statistical analysis was performed using the SPSS software package. Data are expressed as means SE (range). The significance of correlation between parameters was assessed using the Pearson correlation analysis, and P values less than 0.05 (two tailed) were considered to be statistically significant.
Results
Mothers age ranged from 15 to 45 yrs (25.3 ± 6.9), 8 (8.4%) had less than 4 years of schooling; 62 (65.2%) were single. Birth length, weight, WC and BMI were: 40 cm to 53 cm (47.3 ± 2.5), 2100 g to 4320 g (3190 ± 0.466), 26.0 cm to 36 cm (30.9 ± 2.4) and 10.3 Kg/m2 to 23.6 Kg/m2 (14.0 ± 2.1), respectively.
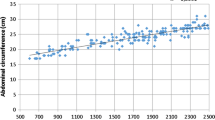
There was a negative correlation between mesenteric fat and WA (r = −0.384; p < 0.001) but not with weight (−0.091; p = 0.380), length (0.105; p = 0.311) and BMI (−0.191; p = 0.064). WA had a positive correlation with birth weight and BMI, respectively, r = 0.325; p < 0.001, and r = 0.304; p = 0.001), but not with length (r = 0.033; p = 0.756).
Discussion
The results indicate a significant negative correlation between AW and mesenteric fat thickness. Small AW in newborns is associated with low birth weight [13] and we showed a positive correlation between those two variables in our study. In adults, AW has a positive correlation with visceral fat [2]. However, under the “thrifty phenotype hypothesis” visceral adiposity in neonates with intrauterine growth restriction may have advantages in the neonatal period but this could persist into adulthood life. Some recent studies in India have demonstrated a higher visceral adiposity and a lower birth weight in Indians neonates compared with English newborns; this phenotype was described as muscle thin but “thinfat” [17, 18]. All these findings are in accordance with developing origins of health and diseases.
Mesenteric fat is a specific portal adipose issue and has a higher lipolytic activity resulting in a high rate of free fatty acids (FFA) production and reduced glucose uptake [4]. The increased FFA from these adipocytes can lead to reduced fat oxidation and ectopic fat deposition in liver and muscle. Furthermore, visceral adipocytes secrete a large number of vasoactive peptides and cytokines all of which can increase atherosclerosis risk: interleukin-6, tumor necrosis factor, angiotensin II, plasminogen activator inhibitor-1, etc. [3]. Besides mesenteric fat thickness showing independent risk associated with fatty liver, is also a predictor for diabetes [6].
For our knowledge, it is the first time that mesenteric fat thickness is used in newborns. This method is approach applicable and reliable for evaluating the distribution of abdominal adipose tissue. Mesenteric fat thickness measured by ultrasound is an approach applicable and reliable for evaluating the distribution of abdominal adipose tissue in adults and has a strong correlation with cardiovascular risk factors. Given the relatively cheap, noninvasive, technically less demanding nature of ultrasound scan together with its good reproducibility, measurement of mesenteric fat thickness may potentially become a useful imaging tool in the investigation of the early origins of obesity.
There are certain limitations of our study. At first, mesenteric fat thickness in newborns has a lower diameter compared with the thickness of adults and children. This means that ultrasound measurement of mesenteric fat thickness in neonates is more difficult to do than in adults and children. However, the results had a good reliability and one of us (E.J.) is a pediatric ultrasonologist with large experience in neonatology. Secondly, our casuistic had few newborns with low or high birth weight. Maybe the comparison between these two distinct groups could indicate a more stronger negative correlation.
Children, who are born small for gestational age, have a predisposition to accumulate visceral fat in childhood and adulthood. However, it is not yet clear if this predisposition is due to their IUGR or their rapid postnatal catch-up growth. The finding that newborns with smaller WA, which is associated with low birth weight, have more mesenteric fat thickness could indicate that IUGR may be more important than the catch-up in the first months of life in the visceral adiposity development. However, evidence for this suggestion would require demonstration of tracking by birth cohort studies.
References
Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S1–8.
Canoy D. Distribution of body fat and risk of coronary heart disease in men and women. Curr Opin Cardiol. 2008;23:591–8.
Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Eitzman DT. Visceral adipose tissue and atherosclerosis. Curr Vasc Pharmacol. 2009;7:169–79.
Bulcão C, Ferreira SR, Giuffrida FM, Ribeiro-Filho FF. The new adipose tissue and adipocytokines. Curr Diabetes Rev. 2006;2:19–28.
Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–71.
Karagiannides I, Pothoulakis C. Neuropeptides, mesenteric fat, and intestinal inflammation. Ann NY Acad Sci. 2008;1144:127–35.
Liu KH, Chan YL, Chan WB, Kong WL, Kong MO, Chan JC. Sonographic measurement of mesenteric fat thickness is a good correlate with cardiovascular risk factor: comparison with subcutaneous and preperitoneal fat thickness, magnetic resonance imaging and anthropometric indexes. Int J Obes Relat Metab Disord. 2001;27:1267–73.
Liu KH, Chan YL, Chan JC, Chan WB. Association of carotid intima-media thickness with mesenteric, preperitoneal fat subcutaneous fat thickness. Atherosclerosis. 2005;179:299–304.
Liu KH, Chan YL, Chan WB, Chan JC, Chu CW. Mesenteric fat thickness is an independent determinant of metabolic syndrome and identifies subjects with increased carotid intima-media thickness. Diab Care. 2006;29:379–84.
Semiz S, Ozgoren E, Sabir N, Semiz E. Body fat distribution in childhood obesity: association with metabolic risk factors. Indian Pediatr. 2008;45:457–82.
Semiz S, Ozgoren E, Sabir N. Comparison of ultrasonographic and anthropometric methods to assess body fat childhood obesity. Int J Obes (Lond). 2007;31:53–8.
McMillen IC, Rattanatray L, Duffield JA, et al. The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol. 2009;646:71–81.
Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20.
McMillen IC, Rattanatray L, Duffield JA, et al. The early origins of later obesity: pathways and mechanisms. Adv Exp Med Biol. 2009;646:71–81.
Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205–10.
Bienstock JL, Holcroft CJ, Althaus J. Small fetal abdominal circumference in the second trimester and subsequent low maternal plasma glucose after a glucose challenge test is associated with the delivery of a small-for-gestational age neonate. Ultrasound Obstet Gynecol. 2008;31:517–9.
Krishnaveni GV, Veena SR, Chachyamma KJ. Truncal adiposity is present at birth and in early childhood in South Indian Children. Indian Pediatr. 2005;42:527–38.
Kulkarni MI, Mythri HP, Kulkarni AM. “Thinfat” phenotype in newborns. Indian J Pediatr. 2009;76:369–73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, J.G., Farias, M.P., Gazineu, R.M. et al. Waist Circumference and Mesenteric Fat in Neonates: Negative Correlation. Indian J Pediatr 77, 1266–1269 (2010). https://doi.org/10.1007/s12098-010-0179-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-010-0179-x