Abstract
Introduction
The role of DCST1-AS1 has been investigated in several types of cancer, while the role of DCST1-AS1 in glioblastoma (GBM) is unclear. This study aimed to investigate the role of DCST1-AS1 in GBM.
Methods
GBM and paired non-tumor tissues were collected from 62 GBM patients. Expression levels of DCST1-AS1 and miR-29b in paired tissue samples were determined by RT-qPCR. The role of DCST1-AS1 in regulating the methylation of miR-29b was assessed by methylation-specific PCR (MSP). Cell proliferation was analyzed by cell proliferation assay.
Results
It was observed that the upregulation of DCST1-AS1 in GBM predicted poor survival. MiR-29b was downregulated in GBM and inversely correlated with the expression of DCST1-AS1. In GBM cells, overexpression of DCST1-AS1 resulted in the downregulation of miR-29b and the increased methylation level of miR-29b gene. Overexpression of DCST1-AS1 resulted in increased cell proliferation. Moreover, Overexpression of DCST1-AS1 significantly reversed the inhibitory effects of miR-29b on cancer cell proliferation.
Conclusion
DCST1-AS1 may downregulate miR-29b through methylation in GBM to promote cancer cell proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM), also refers to glioblastoma multiforme, is the most malignant form of brain cancer [1]. GBM only affects about 3 per 100,000 adults per year [2]. In spite of the low incidence rate, the high mortality rate of GBM makes it a major cause of cancer deaths [3, 4]. It has been estimated that only 25% GBM patients can survive for longer than 1 year after the initial diagnosis even after active treatment [3, 4]. Therefore, novel therapeutic approaches are needed to improve the survival of GBM patients. However, the unclear molecular mechanism of GBM limits the development of more effective therapy [5, 6].
The occurrence and development of GBM involve multiple signaling pathways [7, 8]. Importantly, increasing knowledge of the functions of molecular players in GBM provides novel targets for the development of targeted therapy, which aims to suppress cancer development by regulating the expression of cancer-related genes [9, 10]. Non-coding RNAs (ncRNAs), such as miRNAs and long (> 200 nt) ncRNAs (lncRNAs), have no protein-coding capacity but can regulate gene expression at multiple levels to participate in cancer biology [11]. In effect, regulating the expression of lncRNAs is a potential target for cancer therapy [12]. However, the functions of most lncRNAs in cancer biology remain unknown DCST1-AS1 has been characterized as an oncogenic lncRNA in liver cancer and breast cancer [13,14,15], while its role in GBM is unknown. Our preliminary RNA-seq analysis revealed the dysregulation of DCST1-AS1 in GBM and its inverse correlation with miR-29b, which plays tumor suppressive roles [16]. This study was, therefore, carried out to explore the interaction between DCST1-AS1 and miR-29b in GBM.
Methods
Specimen collection
A total of 38 male and 24 female GBM patients (age range from 46 to 67 years old, mean age 55.7 ± 5.7 years old) were enrolled at Huangshi Central Hospital between July 2016 and July 2018. This study was approved by the Ethics Committee of the hospital. All GBM patients were newly diagnosed cases and the diagnosis was made based on MRI imaging and histopathological biopsy. Patients complicated with other diseases and who had initiated therapies were excluded. All patients signed the written informed consent. Biopsy was performed on all patients to collect paired GBM and non-tumor tissues. All tissue samples were confirmed by histopathological exam.
Treatment and follow-up
All patients were treated with Temozolomide, while the dosage varied according to the health and disease conditions of the patients. From the day of admission, all patients were visited monthly through telephone for a total of 12 months. All 62 patients completed the follow-up. Patients who died of causes unrelated to GBM were not included in the 62 patients.
GBM cells
Two human GBM cell lines U87 and U251 (ATCC) were used. Cell culture medium was composed of 90% DMEM and 10% FBS. Cells were cultivated in an incubator at 37 °C with 95% humidity and 5% CO2.
Transient transfection
To construct DCST1-AS1 expression vector, the full length of DCST1-AS1 cDNA (NCBI Accession: NR_040772.1) was cloned using the Phusion® DNA Polymerase (NEB) with the following PCR conditions: 98 °C for 1 min, followed by 30 cycles of 98 °C for 10 s, 59 °C for 10 s and 72 °C for 1 min, and then 72 °C for 5 min. After that, the full length cDNA of DCST1-AS1 was inserted into pcDNA3.1 vector (Invitrogen). Negative control (NC) miRNA and the mimic of miR-29b were the products of Sigma-Aldrich (USA). Empty vector (1 µg) and/or miRNA (50 nM) was transfected into 108 cells through lipofectamine 2000 (Invitrogen)-mediated transient transfections. Transfection with empty vector or NC miRNA was also performed to serve as NC group. Cells without transfections were cultivated until the end of transfection and used as the Control (C). Transfected cells were further cultivated for 48 h before use.
RNA preparations
Total RNAs were isolated from paired GBM and non-tumor tissue samples as well as U87 and U251 cells using Ribozol reagent (VWR). Genomic DNAs were removed by incubating RNA samples with gDNA eraser (Takara) at 37 °C for 2 h.
RT-qPCR assay
Reverse transcriptions were performed using QuantiTect Reverse Transcription Kit (QIAGEN) with RNA samples as a template to synthesize cDNAs. With cDNAs as template, SYBR Green Master Mix (Bio-Rad) was used to perform qPCRs with 18S rRNA as endogenous control to measure the expression levels of DCST1-AS1. To analyze the expression levels of mature miR-29b, addition of poly (A) to mature miRNAs, miRNA reverse transcriptions and qPCR reactions were performed using All-in-One™ miRNA qRT-PCR reagent kit (GeneCopoeia). U6 was used as the endogenous control of miR-29b. Three replicate reactions were included in each experiment and data were normalized using 2−ΔΔCT method.
Methylation-specific PCR (MSP)
U87 and U251 cells were subjected to genomic DNA extraction suing PureColumn DNA Extraction Kit (BIORON GmbH). DNA Methylation-Gold™ kit (ZYMO RESEARCH) was used to convert DNA samples. Taq 2X Master Mix (NEB) was used to perform MSP to analyze the methylation of miR-29b gene.
CCK-8 assay
U87 and U251 cells were subjected to cell proliferation assay after transfection. A 96-well cell culture plate (3000 cells in 0.1 ml culture medium per well) was used to cultivate cells at 37 °C. OD values were measured at 450 nm every 24 h for a total of 96 h. At 4 h before the measurement of OD values, CCK-8 solution was added into each well to reach the final concentration of 10%.
Statistical analysis
Three replicate reactions were included in each experiment and data were expressed as mean ± standard deviation (SD) values. Paired t test was used to compare differences between paired tissues. ANOVA Tukey’s test was used to compare differences among multiple groups. The 62 GBM patients were divided into high and low DCST1-AS1 level groups (n = 31, median DCST1-AS1 level in GBM as cutoff value). Survival curves of both groups were plotted based on follow-up data. Log-rank test was used to compare survival curves. Correlations were analyzed by linear regression. P < 0.05 was considered as statistically significant.
Results
Upregulation of DCST1-AS1 in GBM predicted poor survival
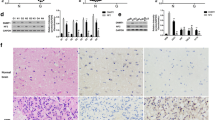
To detect the differential expression of DCST1-AS1 in GBM, the expression levels of DCST1-AS1 in paired GBM and non-tumor tissue samples were measured by RT-qPCR. Compared with non-tumor tissues, GBM tissues exhibited significantly higher expression levels of DCST1-AS1 (Fig. 1a, p < 0.001). Survival curves were plotted for both high and low DCST1-AS1 level groups. Compared with low DCST1-AS1 level groups, patients in high DCST1-AS1 level group showed lower overall survival rate (Fig. 1b). Multivariate Cox regression analysis showed that high expression levels of DCST1-AS1 was an independent prognostic factor for overall survival of GBM patients. The risk ratio was 2.64, 95% CI was 3.33–5.12, and the p value was 0.014.
Upregulation of DCST1-AS1 in GBM predicted poor survival. To detect the differential expression of DCST1-AS1 in GBM, the expression levels of DCST1-AS1 in paired GBM and non-tumor tissue samples were measured by performing RT-qPCR. PCR reactions were repeated three times and mean values were presented (a), ***p < 0.001. The 62 GBM patients were divided into high and low DCST1-AS1 level groups (n = 31) with the median level of DCST1-AS1 in GBM tissues as the cutoff value. Survival curves of both groups were plotted based on follow-up data. Log-rank test was used to compare survival curves (b)
MiR-29b was downregulated in GBM and inversely correlated with the expression of DCST1-AS1
To analyze the differential expression of miR-29b in GBM, the expression levels of miR-29b in paired GBM and non-tumor tissue samples were also measured by RT-qPCR. Compared with non-tumor tissues, GBM tissues exhibited significantly lower levels of miR-29b (Fig. 2a, p < 0.001). Correlation analysis showed that the expression levels of DCST1-AS1 and miR-29b were inversely and significantly correlated with each other across GBM tissues (Fig. 2b), but not in non-tumor tissues (Fig. 2c).
MiR-29b was downregulated in GBM and inversely correlated with the expression of DCST1-AS1. To detect the differential expression of miR-29b in GBM, the expression levels of miR-29b in paired GBM and non-tumor tissue samples were measured by RT-qPCR. PCR reactions were repeated three times and mean values were presented (a), ***p < 0.001. Linear regression was used to analyze the correlations between the expression levels of DCST1-AS1 and miR-29b across GBM tissues (b), but not in non-tumor tissues (c)
Overexpression of DCST1-AS1 led to the downregulation of miR-29b and the increased methylation level of miR-29b gene
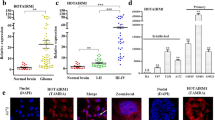
The close correlation between DCST1-AS1 and miR-29b indicated the potential interaction between them. To analyze the interaction between DCST1-AS1 and miR-29b, U87 and U251 cells were transfected with either DCST1-AS1 expression vector or miR-29b mimic, and transfections were confirmed by RT-qPCR (Fig. 3a, p < 0.05). Overexpression of DCST1-AS1 resulted in the downregulation of miR-29b (Fig. 3b, p < 0.05), while overexpression of miR-29b did not affect the expression of DCST1-AS1 (Fig. 3c). MSP was performed to analyze the effects of overexpression of DCST1-AS1 on the methylation of miR-29b. Compared with cell transfected with empty pcDNA3.1 vector, cells transfected with DCST1-AS1 expression vector showed significantly increased methylation of miR-29b gene (Fig. 3d). Therefore, overexpression of DCST1-AS1 may downregulate miR-29b by increasing the methylation of miR-29b gene.
Overexpression of DCST1-AS1 led to downregulation of miR-29b and the increased methylation level of miR-29b gene. To analyze the interaction between DCST1-AS1 and miR-29b, U87 and U251 cells were transfected with either DCST1-AS1 expression vector or miR-29b mimic, and the overexpression of DCST1-AS1 and miR-29b was confirmed by RT-qPCR (a). The effect of overexpression of DCST1-AS1 on miR-29b (b) and the effects of overexpression of miR-29b on DCST1-AS1 (c) were also analyzed by RT-qPCR. MSP was performed to analyze the effects of overexpression of DCST1-AS1 on the methylation of miR-29b (d). Experiments were repeated three times and mean ± SD values were presented. U unmethylation, M methylation; *p < 0.05
DCST1-AS1 promoted GBM cell proliferation through miR-29b
The effects of DCST1-AS1 and miR-29b on the proliferation of U87 and U251 cells were assessed by cell proliferation assay. Compared with the C group, overexpression of DCST1-AS1 resulted in increased cell proliferation rate. Moreover, overexpression of DCST1-AS1 significantly reversed the inhibitory effects of miR-29b on cancer cell proliferation (Fig. 4, p < 0.05). Therefore, DCST1-AS1 may downregulate miR-29b to promote the proliferation of GBM cells.
Discussion
This study assessed the interaction between DCST1-AS1 and miR-29b in GBM. We found that DCST1-AS1 was upregulated in GBM and predicted poor survival. In addition, DCST1-AS1 may downregulate miR-29b through methylation to promote the proliferation of GBM cells.
The function of DCST1-AS1 has been studied in multiple types of cancer, such as liver cancer and breast cancer [13,14,15]. DCST1-AS1 is overexpressed in liver cancer and it interacts with the AKT/mTOR signaling pathway and the miR-1254/FAIM2 axis to promote the migration and proliferation of GBM cells [13, 14]. In breast cancer, DCST1-AS1 is also overexpressed and forms a positive regulation loop with miR-873-5p and MYC to promote cell migration and proliferation [15]. To the best of our knowledge, this study is the first to report the upregulation of DCST1-AS1 in GBM. In addition, overexpression of DCST1-AS1 resulted in increased proliferation rate of GBM cells. Therefore, DCST1-AS1 may promote GBM by enhancing cell proliferation. It is also worth noting that the roles of DCST1-AS1 in the regulation of other behaviors of GBM cells, such as stemness, invasion, apoptosis and migration were also explored, while no significant effects of the overexpression of DCST1-AS1 on these behaviors were observed (data not shown). The possible explanation is that GBMs were all stage IV glioma and the behaviors of GBM cells may be different from other types of cancer cells.
Compared with other types of cancer, the prognosis of GBM is generally poor [3, 4]. In this study, we showed that the high expression levels of DCST1-AS1 before therapy are closely correlated with the poor survival of GBM patients. Therefore, measuring the expression levels of DCST1-AS1 before treatment may assist the prognosis of GBM patients, thereby guiding the determination of treatment approaches and improving survival of patients.
MiR-29b plays tumor-suppressive roles in different types of cancer including GBM [16, 17]. In effect, the downregulation of miR-19b may serve as a diagnostic and prognostic marker for GBM [17]. Consistently, our study also reported the downregulation of miR-29b in GBM. In addition, overexpression of miR-29b led to decreased proliferation rate of GBM [17]. Interestingly, overexpression of DCST1-AS1 resulted in increased methylation of miR-29b, thereby downregulating the expression of miR-29b. For instance, DCST1-AS1 sponges miR-1254 to upregulate FAIM2, thereby promoting the progression of liver cancer [15]. In another study, DCST1-AS1 forms a positive regulation loop with miR-873-5p and MYC to promote the metastasis and proliferation of breast cancer cells [16]. Therefore, DCST1-AS1 may participate in cancer biology mainly by interacting with miRNAs in different ways. It is worth noting that DCST1-AS1 and miR-29b were only inversely correlated with each other across GBM tissues but not in non-tumor tissues. Therefore, the interaction between DCST1-AS1 and miR-29b is likely GBM-specific and certain pathological factors may mediate the interaction between them. Our future studies will try to identify these factors.
In conclusion, DCST1-AS1 is upregulated in GBM and miR-29b is downregulated in GBM. In addition, DCST1-AS1 may downregulate miR-29b through methylation to promote the proliferation of GBM cells.
References
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–50.
Urbańska K, Sokołowska J, Szmidt M, Sysa P. Glioblastoma multiforme—an overview. Contemp Oncol. 2014;18(5):307.
Söderberg-Nauclér C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013;369(10):985.
Smoll NR, Schaller K, Gautschi OP. Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci. 2013;20(5):670–5.
Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829–48.
Mao H, LeBrun DG, Yang J, Zhu VF, Li M. Deregulated signaling pathways in glioblastoma multiforme: molecular mechanisms and therapeutic targets. Cancer Invest. 2012;30(1):48–56.
Jhanwar-Uniyal M, Labagnara M, Friedman M, Kwasnicki A, Murali R. Glioblastoma: molecular pathways, stem cells and therapeutic targets. Cancers. 2015;7(2):538–55.
Itakura H, Achrol AS, Mitchell LA, Loya JJ, Liu T, Westbroek EM, et al. Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci Transl Med. 2015;7(303):303–13838.
Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25.
Touat M, Idbaih A, Sanson M, Ligon K. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28(7):1457–72.
Hauptman N, Glavač D. Long non-coding RNA in cancer. Int J Mol Sci. 2013;14(3):4655–69.
Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253.
Li J, Zhai D, Huang Q, Chen H, Zhang Z, Tan Q. LncRNA DCST1-AS1 accelerates the proliferation, metastasis and autophagy of hepatocellular carcinoma cell by AKT/mTOR signaling pathways. Eur Rev Med Pharmacol Sci. 2019;23(14):6091–104.
Chen J, Wu D, Zhang Y, Yang Y, Duan Y, An Y. LncRNA DCST1-AS1 functions as a competing endogenous RNA to regulate FAIM2 expression by sponging miR-1254 in hepatocellular carcinoma. Clin Sci. 2019;133(2):367–79.
Tang L, Chen Y, Tang X, Wei D, Xu X, Yan F. Long noncoding RNA DCST1-AS1 promotes cell proliferation and metastasis in triple-negative breast cancer by forming a positive regulatory loop with miR-873-5p and MYC. J Cancer. 2020;11(2):311.
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S, et al. MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 2017. https://doi.org/10.1016/j.canlet.2017.03.032.
Zhong F, Huang T, Leng J. Serum miR-29b as a novel biomarker for glioblastoma diagnosis and prognosis. Int J Clin Exp Pathol. 2019;12(11):4106–12.
Funding
The work was supported by the Department of Rehabilitation, Huangshi Central Hospital, Affiliated Hospital of Hubei Polytechnic University.
Author information
Authors and Affiliations
Contributions
YZ supervised the whole study, data analysis, manuscript preparation; SH, YY and XH had data collection and analysis, manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Ethical approval was obtained from the Ethics Committee of the Second Hospital of Shandong University. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients signed the informed consent.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, S., Yao, Y., Hu, X. et al. LncRNA DCST1-AS1 downregulates miR-29b through methylation in glioblastoma (GBM) to promote cancer cell proliferation. Clin Transl Oncol 22, 2230–2235 (2020). https://doi.org/10.1007/s12094-020-02363-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02363-1