Abstract
Background
As a reliable biomarker of breast cancer, breast microcalcification has been reported to be correlated with poor prognosis. Bone morphogenetic protein 2 (BMP-2) plays an important role in microcalcification of breast cancer. Studies in other tissues have shown an association between BMP-2 and AKT/mTOR pathway, while their relationship in breast cancer still remains largely undetermined. To clarify the relationship of these three factors, we collected patients of invasive breast cancer with/without microcalcification and immunohistochemical examination was performed.
Method/patients
A total of 272 patients with primary invasive breast cancer were selected from the First Hospital of China Medical University from January 2010 to January 2012. Immunohistochemical examination of the BMP-2, p-AKT and p-mTOR was performed on 4-µm tissue microarray (TMA) sections. Then, we analyzed the relationship of BMP-2, p-AKT, and p-mTOR and their correlation with disease-free survival (DFS) in breast cancer with/without microcalcification.
Results
We found that breast cancer patients with microcalcification were correlated with HER-2 positive expression and poor prognosis. Immunohistochemical examination showed that the expressions of BMP-2 and p-mTOR were increased in breast cancer with microcalcification and the expressions of BMP-2, p-AKT, and p-mTOR were correlated with each other. Moreover, the high expressions of BMP-2, p-AKT, and p-mTOR were significantly correlated with poor prognosis.
Conclusions
Based on the abovementioned findings, we hypothesized that the high expression of BMP-2 not only played a vital role in the formation of microcalcification, but also activated the AKT/mTOR pathway. Collectively, breast cancer patients with microcalcification were more likely to be resistant to targeted or endocrine therapy and be correlated with poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast microcalcification (< 0.5 mm in diameter) is one of reliable biomarkers of breast cancer, especially in early non-palpable breast cancer. Mammography is now widely used in detection of microcalcification for breast cancer and the mortality of breast cancer is reduced approximately 20%, which is greatly attributed to the early diagnosis by mammography [1]. Several studies have reported that the microcalcification of breast cancer is a poor indicator of long-term clinical outcome [2,3,4,5,6,7,8,9], while the mechanism underlying microcalcification remains largely unexplored. Recent study shows that a subpopulation of breast cancer cells undergoes mesenchymal transition (EMT) and acquires osteoblastic characteristics during microcalcification [10]. Bone morphogenetic protein 2 (BMP-2) is a member of the TGF-β superfamily and it induces matrix mineralization in osteoblast-like cells [11]. Recent findings indicate that BMP-2 is upregulated in breast carcinoma with microcalcification compared with breast carcinoma without microcalcification [10]. Similarly, another study has observed that inoculation of the breast carcinoma cells overexpressing BMP-2 into the rat mammary results in breast tumors with microcalcification [12] and it has been also demonstrated that treatment with recombinant BMP-2 can also induce microcalcification in breast cancer tissue of rats [13]. Based on these findings, BMP-2 may play an important role in microcalcification of breast cancer.
The mammalian target of rapamycin (mTOR) is a highly conserved serine/threonine protein kinase, which is a downstream mediator in the PI3K/AKT signaling pathway [14,15,16]. When such a pathway is activated, AKT is phosphorylated into p-AKT and mTOR is phosphorylated into p-mTOR. The AKT/mTOR pathway has been demonstrated to regulate several cellular functions, including cell growth, survival, angiogenesis, as well as targeted or endocrine therapy resistance [17,18,19,20] and the PI3K/AKT/mTOR pathway is activated in multiple cancers, including breast cancer [21, 22]. Studies have indicated that BMP-2 can regulate chondrocyte maturation [23] and differentiation of osteoclasts [24, 25] through activating the PI3K/AKT pathway. Another two studies in gastric cancer have shown that BMP-2 can accelerate the motility and invasiveness of cancer cells via the activation of AKT [26, 27]. Therefore, BMP-2 may be an upstream regulator of AKT/mTOR pathway in breast cancer. However, it is still unclear whether AKT/mTOR pathway can be regulated by BMP-2 and there is any difference between the expression of BMP-2/AKT/mTOR in breast cancer with and without microcalcification. In the present study, we investigated the potential roles and relationship of BMP-2, p-AKT, and p-mTOR in breast cancer with microcalcification and without microcalcification.
Materials and methods
Patients and tissues
A total of 272 patients with primary invasive breast cancer was selected from the First Hospital of China Medical University from January 2010 to January 2012. Patients with invasive breast cancer of stage I to III who received preoperative mammography were selected in the present study according to the inclusion criteria. The exclusion criteria were set as follows: patients younger than 20 or older than 80 years; patients with distant metastasis at the time of diagnosis; patients with previous history of other malignant neoplasms, including breast cancer; patients who could not undergo radical surgery; and patients who had rare histologic subtype such as inflammatory breast carcinoma.
Patients were followed up for a median of 84 months after their initial cancer surgery. Relevant clinical and pathological parameters were described in Table 1. Archival formalin-fixed paraffin-embedded (FFPE) breast tissues were collected and prepared into tissue microarray (TMA). All of the carcinomas were histologically confirmed as invasive breast cancer according to the criteria of the World Health Organization and the molecular subtypes of breast carcinoma were identified.
This study was approved by the ethics committee of the First Affiliated Hospital (Shenyang, China) and written informed consent was obtained from each patient.
Immunohistochemical staining
Immunohistochemical examination was performed on 4-µm TMA sections. Briefly, following deparaffinization and rehydration, the endogenous peroxidase activity was blocked using 3% H2O2 (reagent A; UltraSensitive™ SP IHC kit; Maxim Biotech Inc., Fuzhou, China). Next, antigen retrieval was performed and normal serum (reagent B; UltraSensitive™ SP IHC kit; Maxim Biotech Inc.) was applied to the sections to block non-specific binding. Sections were then incubated at 4 °C overnight with the primary antibodies, including an anti-rabbit polyclonal antibody against BMP-2 (1:300, ab14933; Abcam, Cambridge, UK), an anti-rabbit polyclonal antibody against AKT (phospho T308) (1:300, ab38449; Abcam, Cambridge, UK) and an anti-rabbit polyclonal antibody against mTOR (phospho S2448) (1:500, ab131538; Abcam, Cambridge, UK). Subsequently, the sections were incubated with the secondary antibody (reagent C; UltraSensitive™ SP IHC kit; Maxim Biotech Inc.) for 15 min, followed by incubation with streptavidin-peroxidase (reagent D, UltraSensitiveTM SP IHC Kit, MXB, Fuzhou, China) and 3,3-diaminobenzidine (DAB) was used to stain the sections. Finally, sections were counterstained with hematoxylin and mounted. Sections incubated with normal rabbit serum (Dako, Carpinteria, CA, USA) served as negative controls. Sections of breast cancer tissue showing strong staining with the respective proteins during antibody optimization served as positive controls.
Evaluation of immunohistochemistry
The results of immunohistochemical staining were independently evaluated and scored by two pathologists in a blinded manner. Cases of disagreement were jointly reviewed to obtain a consensus score. The score was obtained from the average of ten distinct high-power fields (40× objective). The staining was considered positive when cytoplasmic and/or membranous staining was observed in the cancer cells and the staining was evaluated using a semi-quantitative scoring system considering both the extent and intensity. The proportion of stained cells was scored as 0 (no cells stained), 1 (1–10% of cells stained), 2 (11–50% of cells stained), 3 (51–80% of cells stained), or 4 (more than 80% of cells stained). Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). These two parameters were then multiplied, resulting in an individual immunoreactivity score (IRS) ranging from 0 to 12 for every case.
Statistical analysis
Statistical analyses were carried out using SPSS v 19.0. Mann–Whitney U test was performed to assess the independence between two independent samples without any distribution assumption. Pearson correlation coefficient revealed a relationship between two continuous variables. Receiver operating characteristic (ROC) curve analyses were used to select cutoff values (giving the highest combined sensitivity and specificity) to dichotomize the expression scores of BMP-2, p-AKT, and p-mTOR for the end point of disease-free survival (DFS). DFS was estimated using the Kaplan–Meier analyses and recorded from the date of surgery to the date of relapse or last follow-up date. The statistical significance of differential survival was assessed using the log-rank (score) test. Additionally, multivariate Cox regression analysis was performed by taking into account the expressions of BMP-2, p-AKT, p-mTOR, and HER-2, as well as axillary lymph node metastasis and microcalcification. All analyses were two sided and P ≤ 0.05 was considered as statistically significant.
Results
Microcalcification is correlated with the high expression of HER-2 and poor DFS in patients with breast cancer
Among the 272 patients with breast cancer, existing microcalcification was found in 77 patients by preoperative mammography and Table 1 shows the pathologic outcome. We found that 25 cases (32.5%) were HER-2 positive in patients with microcalcification and 30 cases (15.4%) were HER-2 positive in patients without microcalcification. HER-2 positivity was more likely to be associated with microcalcification (χ2 = 9.986, P = 0.002). We also found that there was a significant difference in tumor size between patients with microcalcification and without microcalcification. Patients with microcalcification were correlated with larger tumor size (χ2 = 9.629, P = 0.022) (Table 1).
Kaplan–Meier survival analyses were used to analyze the difference of DFS between patients with microcalcification and without microcalcification. Figure 1a reveals that patients with microcalcification were correlated with poor DFS (χ2 = 5.002, P = 0.025).
Relationship between microcalcification or expression levels of BMP-2, p-AKT, and p-mTOR and patients’ DFS a shows that patients with microcalcification were correlated with poor DFS (P = 0.025), b–d shows that the high expressions of BMP-2, p-AKT, and p-mTOR were significantly correlated with poor DFS (P = 0.001, 0.004, and 0.013, respectively)
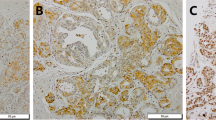
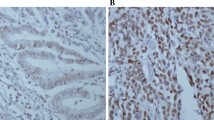
The expression scores of BMP-2 and p-mTOR are significantly increased in breast cancer with microcalcification and correlated with each other
The staining of BMP-2 was found in cytoplasm, nucleus, and cell membrane and such positive staining was found in 74/77 cases (96.1%) and 175/195 cases (89.7%) of tissues with microcalcification and without microcalcification, respectively (Fig. 2a). The staining of p-AKT was detected in both nucleus and cytoplasm. The positive rate of p-AKT was 92.2% (71/77) and 88.7% (173/195) in tissues with microcalcification and without microcalcification, respectively (Fig. 2b). The p-mTOR was expressed in cytoplasm and nucleus, which was present in 72/77 cases (93.5%) and 173/195 cases (88.7%) in tissues with microcalcification and without microcalcification, respectively (Fig. 2c). Figure 3 illustrates the scores and their distributions.
The expression scores of BMP-2, p-AKT, p-mTOR and their distributions. a–c Shows that the expression scores’ median values with interquartile ranges of BMP-2, p-AKT, and p-mTOR in breast cancer tissues with and without microcalcification. d Shows the distribution and correlation of BMP-2, p-AKT, and p-mTOR in breast cancer tissues
The median expression scores of BMP-2, p-AKT, and p-mTOR were 8, 8, and 8 in tissues with microcalcification, respectively, which became 3, 6, and 6 in tissues without microcalcification, respectively. Mann–Whitney U test showed that there was a significant difference in the expressions of BMP-2 and p-mTOR between tissues with microcalcification and without microcalcification. BMP-2 and p-mTOR were significantly increased in tissues with microcalcification (P = 0.000 and P = 0.026, respectively)(Fig. 3a, c). The AKT expression was not significantly different in tissues with and without microcalcification (P = 0.180) (Fig. 3b).
We also examined the correlation of BMP-2, p-AKT, and p-mTOR and found that these three factors were significantly correlated with each other, indicating that BMP-2 might be a regulator of p-AKT and p-mTOR. The correlation coefficient and P value of BMP-2 and p-AKT, BMP-2 and p-mTOR, or p-AKT and p-mTOR were 0.177 and 0.003, 0.164 and 0.007, or 0.172 and 0.004, respectively (Fig. 3d).
High expressions of BMP-2, p-AKT, and p-mTOR are significantly correlated with poor prognosis
Kaplan–Meier survival analyses were performed to assess the differential survival with BMP-2, p-AKT, and p-mTOR. ROC curve analyses were used to dichotomize the expression scores into high and low expression groups. The cutoff values were obtained from the highest combined sensitivity and specificity at the end point of DFS and the cutoff values were selected as follows: BMP, 27, p-AKT, 8.5 and p-mTOR, 7. We found that the high expressions of BMP-2, p-AKT, and p-mTOR were significantly correlated with poor prognosis. (P = 0.001, 0.004, and 0.013, respectively) (Fig. 1b–d).
The univariate factor analysis of other clinicopathological features and DFS
Kaplan–Meier survival analyses were also performed to assess the correlations between differential survival and hormonal receptor, HER-2, age, tumor size, axillary metastasis, surgical method, or chemotherapy regimen. We found that HER-2 and axillary metastasis were the risk factors of the prognosis in breast cancer (P = 0.018 and 0.005, respectively). Other clinicopathological features had no significant correlation with prognosis.
Cox regression analysis
Finally, COX regression analysis was performed by taking into account the statistical significant variables in single factor analysis, including the expressions of BMP-2, p-AKT, p-mTOR, and HER-2, as well as axillary lymph node metastasis and microcalcification. We found that BMP-2, p-AKT, p-mTOR, HER-2, and axillary lymph node metastasis were the independent prognostic factors, with a hazard ratio of 0.454, 0.382, 0.483, 1.380, and 1.588, respectively and with a P value of 0.023, 0.003, 0.028, 0.007, and 0.002, respectively. The microcalcification could not be regarded as an independent prognostic factor for breast cancer (P = 0.881).
Discussion
In the present study, we demonstrated that microcalcification was significantly correlated with poor prognosis in patients with invasive breast cancer (P = 0.025), which was consistent with the results of previous studies [2,3,4,5,6,7,8,9]. However, the formation of mammary microcalcification and its role in breast cancer remains largely unexplored. Previous study [28] has reported that cancers with calcification are more likely to have lymph node metastasis, while our study did not found significant correlation between microcalcification and lymph node metastasis. Moreover, we also found that microcalcification was correlated with HER-2 positivity, which was consistent with the previous studies [9, 29, 30]. It might be partly attributed to the correlation between microcalcification and poor prognosis.
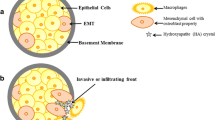
Many studies have shown that BMP-2 may play an important role in the formation of microcalcification [10, 12, 13, 31]. On one hand, BMPs can increase the expression of transient receptor potential channel (TRPC), which may facilitate microcalcification by supplying Ca2+ [32]. On the other hand, they can induce EMT of cancer cells through the AKT pathway and Smad pathway [33,34,35], leading to transdifferentiation of EMT cells to osteoblast-like cells [10]. Previous studies have shown that BMP-2 can regulate the AKT pathway in osseous tissue [23,24,25] and gastric cancer [26, 27], while the relationship between BMP-2 and AKT still remains unknown in breast cancer. Furthermore, it is also unclear whether mTOR and AKT are regulated by BMP-2. To find the roles of these three factors in breast cancer with and without microcalcification and the relationship among them, we performed the immunohistochemical staining to detect their expressions. We found that BMP-2 was significantly increased in tissues with microcalcification (P = 0.000), which was consistent with the previous study [10]. We also demonstrated that the expression of p-mTOR was obviously increased (P = 0.026) in tissues with microcalcification. As expected, these three factors also had a significant correlation with each other, indicating that the high expression of BMP-2 could upregulate the AKT/mTOR pathway and significantly increase the other two factors in breast cancer with microcalcification (Fig. 4). However, the AKT/mTOR pathway is regulated by multiple growth factors [17]. Therefore, further studies are required to clarify the deep relationship between BMP-2 and AKT/mTOR pathway.
Proposed molecular mechanism for BMP-2 in breast cancer tissues with microcalcification. On one hand, BMP-2 activated the Smad pathway and increased the expression of Ca2+ channel (TRPC), which might facilitate microcalcification by supplying Ca2+. On the other hand, BMP-2 could upregulate the AKT/mTOR pathway, by which patients with microcalcification were more likely to resist targeted or endocrine therapy
Studies have shown that BMPs can promote invasion and migration of breast cancer cells [36,37,38,39] presumably by inducing EMT [33,34,35]. Recent literature has also indicated a positive association between serum BMP and cancer metastasis [40]. In our present study, we demonstrated that the high expression of BMP-2 was significantly associated with poor prognosis (P = 0.001) and BMP-2 expression was significantly increased in tissues with microcalcification (P = 0.000), which might partly explain the poor prognosis of breast cancer with microcalcification. The AKT/mTOR pathway has been shown to play a critical role in cell growth [17, 18]. Previous study has shown that mTOR is correlated with resistance to HER-2 therapies (trastuzumab) in breast cancer [19] and it has been demonstrated to be correlated with endocrine therapy resistance [20]. In our study, there was a significant association between the high expression of AKT/mTOR pathway and poor prognosis (P = 0.004 and 0.013). We also found that the AKT/mTOR pathway was activated by BMP-2 in breast cancer with microcalcification as previously described. This finding indicated that breast cancer patients with microcalcification might be more likely to resist targeted or endocrine therapy due to the high expression of AKT/mTOR pathway. However, microcalcification could not be regarded as an independent prognostic factor of breast cancer in Cox regression analysis (P = 0.881), which might be attributed to that there were some other mechanisms involved in the formation of microcalcification.
Conclusions
Collectively, microcalcification was a poor prognostic factor for breast cancer patients and BMP-2 might play an important role in the form of microcalcification. Moreover, BMP-2 was significantly increased in tissues with microcalcification, which could activate AKT/mTOR pathway at the same time. This finding might partly explain the correlation between microcalcification and a poor prognosis.
References
Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–40. https://doi.org/10.1038/bjc.2013.177.
Tabár L, Chen H-H, Duffy SW, Yen MF, Chiang CF, Dean PB, et al. A novel method for prediction of long-term outcome of women with T1a, T1b, and 10–14 mm invasive breast cancers: a prospective study. Lancet. 2000;355(9202):429–33. https://doi.org/10.1016/s0140-6736(00)82008-5.
Thurfjell E, Thurfjell MG, Lindgren A. Mammographic finding as predictor of survival in 1–9 mm invasive breast cancers. Worse prognosis for cases presenting as calcifications alone. Breast Cancer Res Treat. 2001;67(2):177–80.
Gajdos C, Tartter PI, Bleiweiss IJ, Hermann G, de Csepel J, Estabrook A, et al. Mammographic appearance of nonpalpable breast cancer reflects pathologic characteristics. Ann Surg. 2002;235(2):246–51.
Evans AJ, Pinder SE, Snead DR, Wilson AR, Ellis IO, Elston CW. The detection of ductal carcinoma in situ at mammographic screening enables the diagnosis of small, grade 3 invasive tumours. Br J Cancer. 1997;75(4):542–4.
Zunzunegui RG, Chung MA, Oruwari J, Golding D, Marchant DJ, Cady B. Casting-type calcifications with invasion and high-grade ductal carcinoma in situ: a more aggressive disease? Arch Surg. 2003;138(5):537–40. https://doi.org/10.1001/archsurg.138.5.537.
Bennett RL, Evans AJ, Kutt E, Record C, Bobrow LG, Ellis IO, et al. Pathological and mammographic prognostic factors for screen detected cancers in a multi-centre randomised, controlled trial of mammographic screening in women from age 40 to 48 years. Breast. 2011;20(6):525–8. https://doi.org/10.1016/j.breast.2011.05.008.
Holmberg L, Wong YN, Tabar L, Ringberg A, Karlsson P, Arnesson LG, et al. Mammography casting-type calcification and risk of local recurrence in DCIS: analyses from a randomised study. Br J Cancer. 2013;108(4):812–9. https://doi.org/10.1038/bjc.2013.26.
Ling H, Liu ZB, Xu LH, Xu XL, Liu GY, Shao ZM. Malignant calcification is an important unfavorable prognostic factor in primary invasive breast cancer. Asia-Pacific J Clin Oncol. 2013;9(2):139–45. https://doi.org/10.1111/j.1743-7563.2012.01572.x.
Scimeca M, Giannini E, Antonacci C, Pistolese CA, Spagnoli LG, Bonanno E. Microcalcifications in breast cancer: an active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer. 2014;14:286. https://doi.org/10.1186/1471-2407-14-286.
Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, et al. Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. J Bone Min Res. 2006;21(5):674–83. https://doi.org/10.1359/jbmr.020603.
Liu F, Bloch N, Bhushan KR, De Grand AM, Tanaka E, Solazzo S, et al. Humoral bone morphogenetic protein 2 is sufficient for inducing breast cancer microcalcification. Mol Imaging. 2008;7(4):175–86.
Inoue K, Liu F, Hoppin J, Lunsford EP, Lackas C, Hesterman J, et al. High-resolution computed tomography of single breast cancer microcalcifications in vivo. Mol Imaging. 2011;10(4):295–304. https://doi.org/10.2310/7290.2010.00050.
Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278–87. https://doi.org/10.1200/JCO.2008.20.0766.
Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–34. https://doi.org/10.1038/nrc1974.
Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg Oncol Clin N Am. 2013;22(4):641–64. https://doi.org/10.1016/j.soc.2013.06.008.
Alayev A, Holz MK. mTOR signaling for biological control and cancer. J Cell Physiol. 2013;228(8):1658–64. https://doi.org/10.1002/jcp.24351.
Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. https://doi.org/10.1038/nature10912.
Brady SW, Zhang J, Tsai MH, Yu D. PI3K-independent mTOR activation promotes lapatinib resistance and IAP expression that can be effectively reversed by mTOR and Hsp90 inhibition. Cancer Biol Ther. 2015;16(3):402–11. https://doi.org/10.1080/15384047.2014.1002693.
Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29(33):4452–61. https://doi.org/10.1200/JCO.2010.34.4879.
Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA, et al. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther. 2011;10(6):1093–101. https://doi.org/10.1158/1535-7163.MCT-10-1089.
McCubrey JA, Davis NM, Abrams SL, Montalto G, Cervello M, Libra M, et al. Targeting breast cancer initiating cells: advances in breast cancer research and therapy. Adv Biol Regul. 2014;56:81–107. https://doi.org/10.1016/j.jbior.2014.05.003.
Kakoi H, Maeda S, Shinohara N, Matsuyama K, Imamura K, Kawamura I, et al. Bone morphogenic protein (BMP) signaling up-regulates neutral sphingomyelinase 2 to suppress chondrocyte maturation via the Akt protein signaling pathway as a negative feedback mechanism. J Biol Chem. 2014;289(12):8135–50. https://doi.org/10.1074/jbc.M113.509331.
Mandal CC, Das F, Ganapathy S, Harris SE, Choudhury GG, Ghosh-Choudhury N. Bone morphogenetic protein-2 (BMP-2) activates NFATc1 transcription factor via an autoregulatory loop involving Smad/Akt/Ca2 + signaling. J Biol Chem. 2016;291(3):1148–61. https://doi.org/10.1074/jbc.M115.668939.
Lauzon MA, Drevelle O, Daviau A, Faucheux N. Effects of BMP-9 and BMP-2 on the PI3K/Akt Pathway in MC3T3-E1 preosteoblasts. Tissue Eng Part A. 2016;22(17–18):1075–85. https://doi.org/10.1089/ten.TEA.2016.0151.
Kang MH, Kim JS, Seo JE, Oh SC, Yoo YA. BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res. 2010;316(1):24–37. https://doi.org/10.1016/j.yexcr.2009.10.010.
Kang MH, Oh SC, Lee HJ, Kang HN, Kim JL, Kim JS, et al. Metastatic function of BMP-2 in gastric cancer cells: the role of PI3K/AKT, MAPK, the NF-kappaB pathway, and MMP-9 expression. Exp Cell Res. 2011;317(12):1746–62. https://doi.org/10.1016/j.yexcr.2011.04.006.
Iwasaki Y, Fukutomi T, Akashi-Tanaka S, Nanasawa T, Tsuda H. Axillary node metastasis from T1N0M0 breast cancer: possible avoidance of dissection in a subgroup. Jpn J Clin Oncol. 1998;28(10):601–3.
Sun SS, Zhang B, Zhao HM, Cao XC. Association between mammographic features and clinicopathological characteristics in invasive ductal carcinoma of breast cancer. Mol Clin Oncol. 2014;2(4):623–9. https://doi.org/10.3892/mco.2014.297.
Nie Z, Wang J, Ji XC. Microcalcification-associated breast cancer: HER2-enriched molecular subtype is associated with mammographic features. Br J Radiol. 2018. https://doi.org/10.1259/bjr.20170942.
Cox RF, Hernandez-Santana A, Ramdass S, McMahon G, Harmey JH, Morgan MP. Microcalcifications in breast cancer: novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br J Cancer. 2012;106(3):525–37. https://doi.org/10.1038/bjc.2011.583.
Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong N, et al. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2013;49(2):212–20. https://doi.org/10.1165/rcmb.2012-0051OC.
Liao A, Wang W, Sun D, Jiang Y, Tian S, Li J, et al. Bone morphogenetic protein 2 mediates epithelial-mesenchymal transition via AKT and ERK signaling pathways in gastric cancer. Tumour Biol. 2015;36(4):2773–8. https://doi.org/10.1007/s13277-014-2901-1.
Richter A, Valdimarsdottir L, Hrafnkelsdottir HE, Runarsson JF, Omarsdottir AR, Ward-van Oostwaard D, et al. BMP4 promotes EMT and mesodermal commitment in human embryonic stem cells via SLUG and MSX2. Stem Cells. 2014;32(3):636–48. https://doi.org/10.1002/stem.1592.
Xu T, Yu CY, Sun JJ, Liu Y, Wang XW, Pi LM, et al. Bone morphogenetic protein-4-induced epithelial-mesenchymal transition and invasiveness through Smad1-mediated signal pathway in squamous cell carcinoma of the head and neck. Arch Med Res. 2011;42(2):128–37. https://doi.org/10.1016/j.arcmed.2011.03.003.
Clement JH, Raida M, Sanger J, Bicknell R, Liu J, Naumann A, et al. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol. 2005;27(2):401–7.
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27(49):6322–33. https://doi.org/10.1038/onc.2008.232.
Montesano R. Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun. 2007;353(3):817–22. https://doi.org/10.1016/j.bbrc.2006.12.109.
Ketolainen JM, Alarmo EL, Tuominen VJ, Kallioniemi A. Parallel inhibition of cell growth and induction of cell migration and invasion in breast cancer cells by bone morphogenetic protein 4. Breast Cancer Res Treat. 2010;124(2):377–86. https://doi.org/10.1007/s10549-010-0808-0.
Choi YJ, Kim ST, Park KH, Oh SC, Seo JH, Shin SW, et al. The serum bone morphogenetic protein-2 level in non-small-cell lung cancer patients. Med Oncol. 2012;29(2):582–8. https://doi.org/10.1007/s12032-011-9852-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Ethical approval
The experimental protocol was approved by the research ethics committee of First Affiliated Hospital, China Medical University.
Informed consent
All patients involved in this study were aware of the study and signed informed consent forms.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Gu, M., Jiang, H. et al. BMP-2 upregulates the AKT/mTOR pathway in breast cancer with microcalcification and indicates a poor prognosis. Clin Transl Oncol 22, 1263–1271 (2020). https://doi.org/10.1007/s12094-019-02248-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02248-y