Abstract
Background and purpose
Emerging evidence suggests that one of the main reasons of chemotherapy treatment failure is the development of multi-drug resistance (MDR) associated with cancer stem cells (CSCs). Our aim is to identify a therapeutic strategy based on MDR-reversing agents.
Materials and methods
CSC-enriched Ehrlich carcinoma (EC) cell cultures were prepared by drug-resistant selection method using different concentrations of cisplatin (CIS). Cell cultures following drug exposure were analyzed by flow cytometry for CSC surface markers CD44+/CD24−. We isolated murine bone marrow-derived dendritic cells (DCs) and then used them to prepare CSC–DC vaccine by pulsation with CSC-enriched lysate. DCs were examined by flow cytometry for phenotypic markers. Solid Ehrlich carcinoma bearing mice were injected with the CSC–DC vaccine in conjunction with repeated low doses of CIS. Tumor growth inhibition was evaluated and tumor tissues were excised and analyzed by real-time PCR to determine the relative gene expression levels of MDR and Bcl-2. Histopathological features of tumor tissues excised were examined.
Results and conclusion
Co-treatment with CSC–DC and CIS resulted in a significant tumor growth inhibition. Furthermore, the greatest response of downregulation of MDR and Bcl-2 relative gene expression were achieved in the same group. In parallel, the histopathological observations demonstrated enhanced apoptosis and absence of mitotic figures in tumor tissues of the co-treatment group. Dual targeting of resistant cancer cells using CSC–DC vaccine along with cisplatin represents a promising therapeutic strategy that could suppress tumor growth, circumvent MDR, and increase the efficacy of conventional chemotherapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemo-resistance is one of the main causes of tumor relapse and treatment failure in cancer patients. It is also often associated with more aggressive cancers and metastasis [1]. Accumulating evidences suggest that a residual subpopulation of cells remaining following chemotherapy is responsible for chemo-resistance and tumor recurrence and is termed as cancer stem cells (CSCs) [2]. This subpopulation of cells possesses stem cells like character with high tumorigenic ability and high resistance to most chemotherapeutic agents as well as increased anti-apoptotic activity [3, 4].
Consequently, failure of chemotherapeutic regimens to completely eradicate tumor is due to their inability to target this small subpopulation (CSCs) which is suggested to be the principal factor of tumor metastasis and relapse. Treatments that are directed more specifically toward CSCs represent an attractive strategy for effective anti-tumor responses [4, 5].
Previous studies have identified the CD44+/CD24− phenotype as CSC surface markers in an Ehrlich carcinoma (EC) cell line. In addition, these CSCs’ population exhibited stem cells characteristics such as self-renewal, high tumorigenicity, and chemo-resistance [6, 7].
Cisplatin is one of the most potent chemotherapeutic agents, which has been reported to be effective in treating different types of cancers, such as breast cancer, head and neck cancer, and gastric cancer [8,9,10]. Unfortunately, its use is limited due to its adverse effects in normal tissues on long-term administration and the development of high drug resistance [11, 12]. Prior study suggested that the cisplatin resistance is driven by CSCs. Therefore, targeting CSCs is critical for increasing sensitivity of tumor cells to cisplatin treatment. Limited data concerning CSCs genetics make it difficult to target these cells’ population [12].
In contrast to chemotherapies, cancer immunotherapy aimed to modulate the immune system to induce anti-tumor responses generally without severe side effects. Cancer vaccination based on loading dendritic cells (DCs) with specific tumor antigens is an active immunotherapy which results in immunological responses against tumor cells that escape immune recognition [13].
In this study, we used chemotherapeutic drug selection method to enrich drug resistant cells with CSC phenotype (CD44+/CD24−) from parental EC cell line in vitro to be subsequently used as a source enriched in stem cells for pulsing murine bone marrow-derived dendritic (DC) cells. The current study aimed to investigate the therapeutic efficacy of immunization using CSC-based DC vaccine either as a single treatment or in combination with repetitive low doses of cisplatin to overcome cisplatin related chemo-resistance.
Materials and methods
Animal and ethics statement
Female Swiss albino mice were obtained from National Research Center (Cairo, Egypt). Mice were housed in specific pathogen-free facility at the Faculty of Pharmacy, Tanta University and maintained 2 weeks for acclimatization in plastic cages under standard conditions and provided with standard rodent pellet diet and water ad libitum. The mice used for the experiments were at the age of 4–6 weeks and weighed between 22 and 25 g. All animal experiments were approved by the Institutional Research Ethical Committee of Faculty of Pharmacy, Tanta University.
Cell line
Ehrlich carcinoma (EC) is transplantable murine cancer cell line that is derived from aggressive, malignant, and highly proliferating mouse breast adenocarcinoma [14]. EC cell line was purchased from National Cancer Institute (Cairo, Egypt) and was maintained in the ascitic form in the peritoneal cavity of a mouse by sequential passaging via weekly i.p. injection of 2.5 × 106 viable EAC cells/mouse suspended in 0.2 mL PBS [15]. The cell count and viability was assessed by trypan blue dye exclusion method [16], and the cell viability was found to be more than 99%.
Cell culture and selection of cisplatin-resistant cell populations
CSCs are characterized by being more resistant to anticancer drugs than the bulk of tumor cells [17]. Cisplatin-resistant CSCs were selected from parental EC cell line based on their intrinsic resistance to cisplatin. EC cells were cultured in freshly prepared complete medium consisting of RMPI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 mg/mL streptomycin, 100 U/mL penicillin, and 0.5 mg/mL fungizone as previously described by Salem et al. [18].
After 24 h of culture, EC cells reached confluence and were supplemented with 0, 10, 20, 30, 40, and 50 μg/mL cisplatin (CIS) as previously reported [19]. Each concentration was repeated in three different tissue culture flasks. Cell count and viability was estimated 72 h later to determine the highest CIS concentration that EC cells could survive and grow. These drug-resistant cells were harvested by incubating with 0.25% Trypsin/EDTA and subsequently analyzed for stem cell-associated surface markers CD44+/CD24− by flow cytometry.
CSCs surface markers analysis by flow cytometry
CD44-PE and CD-24 FITC anti-mouse antibodies (Miltenyi Biotec Gmb, Germany) were used for flow cytometric analysis of CSC-associated surface markers. Briefly, cells from parental EC and cisplatin-treated cultures were incubated with the above-mentioned fluorescent-conjugated monoclonal antibodies at 4 °C for 30 min. and analyzed by BD FACSCanto™ II flow cytometer (BD biosciences, USA). The obtained data were analyzed by the FlowJo® software package (Tree Star Inc., USA). EC cells were then cultured in medium supplemented with the optimal selected CIS concentration that showed the highest percentage of CD44+/CD24− cells. Isolated CIS-resistant cells population enriched in CSCs—identified as CD44+/CD24−—were harvested for subsequent cell lysate preparation.
Preparation of CSC-pulsed DCs’ vaccine
To prepare enriched CSC lysate, the harvested CIS-resistant cells enriched in stem cell populations were washed twice and suspended in sterile PBS at a concentration of 5 × 106 cells per mL. Cells were lysed and disrupted by four rapid freeze–thaw cycles in 37 °C water bath and − 80 °C. After centrifugation for 10 min at 3000 rpm at 4 °C, supernatant cell lysates were collected and stored as aliquots at − 80 °C.
Bone marrow (BM)-derived DCs were generated as previously described [19]. Briefly, bone marrow of (n = 15) healthy mice was flushed from femur and tibia bones under sterile conditions. The collected BM cells were cultured—after being depleted of RBCs using ammonium chloride potassium (ACK) lysis buffer (Lonza, Germany)—in fresh complete RPMI-1640 with 10% FBS medium supplemented with 20 ng/mL IL-4 and 20 ng/mL GM-CSF at a concentration of 1 × 106 cells/mL. Fresh medium supplemented with the same cytokines was added every 3 days. On day 6, PBS (unloaded DCs) or lysates of enriched CSCs (CSC–DCs) were added to DCs culture medium at a ratio of 1:1 equivalent cell ratio [20]. After incubation for 24 h, 20 ng/mL of maturation stimulus, TNF-α was added to the culture medium for additional 24 h.
Generated mature DCs pulsed with resistant CSCs lysates were harvested and aliquots were directly stained using fluorescent-conjugated monoclonal antibodies, including anti-CD11c, CD83, and CD86, for 30 min at 4 °C in the dark. Cells were then analyzed using FACSCanto™ II flow cytometer (BD biosciences, USA) with FlowJo® software (Tree Star Inc., USA).
Tumor induction and treatment strategy
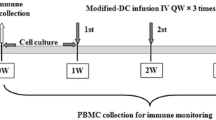
Solid Ehrlich carcinoma (SEC) was induced in Swiss albino mice (n = 36 mice) by injecting viable EAC cells (2 × 106 cells) subcutaneously into the right thigh of the lower limb. A palpable solid tumor mass was formed 14 days post-inoculation, after which SEC-bearing mice were randomly divided into six groups of six mice each. The untreated control group (control) was i.p. (intraperitoneal) treated with PBS; The second group was i.p. treated with CIS at a dose of 2 mg/kg in five cycles, which were on day 14, 18, 22, 26, and 30 (CIS); The third and fourth groups were both i.p. treated with single CIS dose of 2 mg/kg on day 14 and then unloaded DCs (DC group) or CSC-pulsed DCs (CSC–DC group) were intravenously injected in mice tail twice on day 15 and 23, respectively. The last two groups were treated in a similar manner as the third and fourth groups, but were co-treated with CIS 2 mg/kg on days 14, 18, 22, 26, and 30. Treatment protocol is illustrated in Fig. 1.
Treatment strategy timeline. Solid tumors were induced in mice by subcutaneous injection of 2.5 × 106 cells on day 1. Mice in treated groups received small dose of cisplatin (CIS) (2 mg/kg) by intraperitoneal injection on day 14. Immunized groups (C, D, E & F) received either DCs or CSCs pulsed DCs by intravenous injection in tail vein on days 15 and 23. Mice were sacrificed on day 31 and tumor masses were excised to perform further analysis. The studied groups are shown as follows: a control (untreated group); b CIS group: mice in this group were injected with CIS (2 mg/kg) intraperitoneally on days 14, 18, 22, 26, and 30 in five cycles with 3-day intervals. c, d DC and CSC–DC groups, respectively: mice in these two groups were intraperitoneally treated with small dose of CIS (2 mg/kg) as a single dose on day 14 once a solid tumor appeared and then vaccinated by intravenous injection with either DCs (group C) or CSC-pulsed DCs (group D) twice on days 15 and 23; e, f CIS + DC and CIS + CSC–DC groups, respectively: mice in the last two groups received CIS (2 mg/kg) intraperitoneally on days 14, 18, 22, 26, and 30 as five cycles with 3-day intervals and vaccinated by intravenous injection with either DCs (group E) or CSC-pulsed DCs (group F) twice on days 15 and 23
The tumor dimensions were measured using a caliper on day 14 once a palpable mass formed, and then, the same measurement was repeated twice weekly. Tumor inhibition rate (TIR) was calculated according to Salem et al. using the following formula: TIR = [(average tumor volume of control untreated group − average tumor volume of treated group) × 100/average tumor volume of control untreated group] [14].
Histopathological examination of tumor tissues
Tumor tissues were excised, fixed in 10% formalin, dehydrated, and then embedded in paraffin in form of blocks. Sections of 4 mm were prepared using microtome (Leica RM2135, Germany), dewaxed, and stained with Hematoxylin and Eosin (H&E) stain to be examined for the histopathological features using electric microscope equipped with digital camera (Olympus Electron Microscope, Japan).
Total RNA extraction, cDNA synthesis, and real-time PCR analysis
Tumor tissues from control and treated groups were disrupted and homogenized using TissueLyser II (Qiagen, Germany). Total RNA was extracted from homogenized tumor tissue samples using Simply P total RNA extraction kit (BioFlux®, China) according to the manufacturer’s protocol. 1 µg of total RNA was used for synthesis of cDNA using Quantitect reverse transcription kit (Qiagen®, Germany) according to the manufacturer’s protocol. The generated cDNA was used directly in real-time PCR which was performed using SensiFAST™ SYBR No-ROX Kit (Bioline, Germany). The relative expression levels of MDR and Bcl-2 genes were determined using the following primer sequences (Invitrogen, USA): for Bcl-2, forward: 5′- CGGGAGAACAGGGTATGA-3′ (F), reverse: 5′- CAGGCTGGAAGGAGAAGAT-3′ (R); for MDR, forward: 5′-ATCAACTCGCAAAAGCATCC-3′ (F), reverse: 5′- ATTCAACTTCAGGATCCGC-3′ (R).
Thermal cycling conditions comprised an initial polymerase enzyme activation at 95 °C for 2 s, then 40 cycles of 95 °C for 5 s, annealing at 55 °C for 10 s, and extension at 72 °C for 20 s. Results were calculated and normalized relative to the reference GAPDH control gene using the Microsoft Excel program. The relative expression levels were calculated relative to GAPDH using the 2−ΔΔCT method [21]. Data were expressed as relative fold differences in target gene expression and represent the average of three independent experiments.
Statistical analysis
Data were analyzed using Statistical Package for Social Science (SPSS) version 19.0 and were expressed as mean ± SD. To determine significant differences between two groups, P values were calculated by unpaired student t test. Comparisons between three groups or more were performed with one-way analysis of variance (ANOVA). P values less than 0.05 were considered statistically significant. Survival analysis was done by Kaplan–Meier using log-rank test.
Results
Enrichment and identification of cisplatin-resistant cell populations
Ehrlich carcinoma cell cultures treated with 10–50 μg/mL CIS were proliferated slowly compared to parental untreated cell cultures. Cell cultures treated with 50 μg/mL CIS showed increased the rate of cell death after 72 h of drug exposure, and only a few cells were able to survive and continued to grow forming CIS-resistant cell populations which could tolerate and resist the applied high CIS dose (Fig. 2a, b).
Selection of cisplatin-resistant cell populations with CSCs’ phenotype. Morphological appearance of EC parental cell culture a before and b 72 h after treatment with 50 μg/mL cisplatin. c Impact of applying increasing cisplatin concentrations (10–50 μg/mL) on the percentage of CD44+/CD24− cells. CD44 and CD24 were detected using fluorochrome-conjugated anti-mouse antibodies. Data are presented as mean ± SD. a: significant versus control group. Triplicate experiments were performed. **P < 0.01. EC Ehrlich carcinoma; CIS cisplatin; CD cluster of differentiation. Concentrations of cisplatin are expressed as μg/mL
We then assessed the percentage of CSC-associated cell surface markers identified as CD44+/CD24− in different CIS-treated cultures as well as parental EC cell cultures using flow cytometry analysis to investigate whether these resistant cell populations possess CSCs’ surface markers. Treatment of EC cell line with low concentrations of CIS (10–30 μg/mL) resulted in weak inhibition of cell growth and showed non-significant increase in the expression of CD44+/CD24− cells compared to parental cell culture, while higher concentrations (40 and 50 μg/mL) showed a significant increase (P < 0.01) in the expression of CD44+/CD24− cells compared to parental untreated cell cultures.
Treatment with CIS (50 μg/mL) showed the highest percentage of CD44+ CD24− expression among other CIS-treated cultures (Fig. 2c). Our results demonstrated that treatment with 50 μg/mL CIS not only selected cisplatin-resistant cell populations but also were enriched in cancer cells with CSCs characteristics. EC cells were then cultured in complete medium supplemented with 50 μg/mL CIS to develop resistant cell populations enriched in CSCs to be used in further experiments.
Production of functional mature dendritic cells (DCs) derived from mice bone marrow (BM) mononuclear cells
Bone marrow cells were cultured in RPMI medium with GM-CSF and IL-4 to generate immature DCs which is then pulsed with cell lysates enriched in CIS-resistant cells with CSCs phenotype followed by activation using TNF-α. Morphological observation showed formation of dendrites (Fig. 3c). Mature DCs expressed the typical phenotypes including the presence of CD11c, CD83, and CD86 (Fig. 3a, b). As shown in Fig. 3, mature DCs expressed CD11c +/CD83 + (58.4 ± 3.6%) in DC group, and (57.7 ± 1.7%) in DC pulsed with CSC-enriched cell lysates (CSC–DC) group. Similarly, the percentage of CD11c +/CD86 + DCs was 58.9 ± 4.8% in DC group, and 57.3 ± 2.2% in CSC-pulsed DC group as detected by flow cytometric analyses. The generated DCs either DC or CSC–DC groups showed similar phenotypical characteristics of mature DCs.
Generation of functional bone marrow-derived dendritic cells (DCs) expressing phenotypical characteristics of mature DCs. Dot plot flow cytometry analysis of phenotypes of DC (a) and DC pulsed with CSCs lysates (b). Data represent the percentage of DCs identified as CD11c +/CD86 + and CD11c +/CD83 + . Triplicate experiments were performed. Data are presented as mean ± SD. Percentages indicate the percent of cells in that quadrant. c Microscopic images showing the morphological features of generated mature dendritic cells on day 9 of cell culture. The figure shows mature CSC–DCs with branched projections and extended morphology (Original magnification 40X)
Impact of vaccination with CSC–DC on tumor inhibition rate (TIR)
Tumor growth was significantly inhibited in all treated groups (P < 0.001) compared with untreated control group. The inhibition rate was significantly increased in DC (55.7%; P < 0.05), CIS + DC (56.7%; P < 0.01), and CSC–DC (66.7%; P < 0.001) compared to (40.9%) CIS group. The maximum increase in tumor inhibition rate (82%) was evident in CIS + CSC–DC group, as shown in Fig. 4a. Vaccination of SEC-bearing mice with CSC–DC showed a significant increase in TIR compared to either DC or CIS + DC-treated groups. Moreover, this effect was augmented by the simultaneous administration of repeated low doses of cisplatin. Figure 4b shows comparing tumor masses after sacrificing SEC-bearing mice at the end of the study. The administration of CSC–DC either alone or in combination with CIS showed slightly better survival relative to other treated groups where it did not result in death, as shown in Fig. 4c.
Effect of immunization using CSC–DC-based vaccine either as single treatment or in combination with cisplatin on tumor inhibition rate a in SEC-bearing mice. b Gross tumor images of different studied groups on day 31 of the in vivo study. The figure shows comparing tumor sizes before excision from different studied groups on day 31 after sacrificing mice. Arrows indicate the developed tumor masses. c Kaplan–Meier survival curve of various treated groups as described on the chart. Data are presented as the mean ± SD. #P < 0.05, *P < 0.01, **P < 0.001. a Significant increase versus control group, b Significant increase versus CIS-treated group, c Significant increase versus DC- treated group, d Significant increase versus DC + CIS-treated group, e Significant increase versus CSC–DC-treated group. SEC: solid Ehrlich carcinoma, CIS: cisplatin, DC: dendritic cells treated—group, CSC–DC: CSC-pulsed dendritic cells, DC + CIS: combined treatment with DC and cisplatin, CSC–DC + CIS: combined treatment with CSC–DC and cisplatin
Histopathological observations of tumor sections from SEC-bearing mice
The histopathological findings are presented in Fig. 5. Histopathological sections from tumor tissues of untreated control group showed typical neoplastic changes such as presence of large number of multinucleated cells with frequent mitotic figures as well as minimal necrotic areas (Fig. 5a). Tumor tissues from SEC-bearing mice treated with low doses of CIS showed necrotic areas and isolated mitotic figures. Necrotic tissues showed amorphous eosinophilic mass and loss of the plasma membrane integrity and the cell nucleus (Fig. 5b).
Photomicrographs of hematoxylin and eosin-stained tumor sections from SEC-bearing mice. Sections refer to: a untreated tumor control group showing the presence of mitotic figures (encircled by yellow circles), b CIS-treated group showing necrotic areas (N), c DC-treated group, d CIS + DC co-treated group (original magnification 200×), e CSC–DC group showing the presence of some apoptotic figures (arrow) (400×). f CSC–DC + CIS co-treated group showing the absence of mitotic figures and an increase in apoptotic bodies (arrow) (400×)
Tumor sections from DC and CIS + DC groups showed a few apoptotic residual bodies and minimal areas of necrosis (Fig. 5c, d). Tumor sections from CSC–DC-treated group showed an increase in apoptotic bodies as well as a decrease in the number of multinucleated cells (Fig. 5e). Combination therapy with CSC–DC and CIS further increased the apoptotic bodies compared with other tumors treated groups with the absence of mitotic figures indicating efficient apoptotic death responses (Fig. 5f).
Effect of vaccination with CSC–DC on MDR and Bcl-2 gene expression
The presence of CSCs is thought to be responsible for developing MDR and is associated with resistance to apoptosis [1]. In the current study, we determined the relative gene expression levels of MDR and Bcl-2 in tumor tissues from all studied groups. As shown in Fig. 6a, MDR gene expression was significantly downregulated in CIS, DC, CIS + DC, CSC–DC or CIS + CSC–DC groups (↓ 0.49 ± 0.02, ↓ 0.32 ± 0.01, ↓ 0.15 ± 0.01, ↓ 0.16 ± 0.07, and ↓ 0.02 ± 0.004 fold) compared to control group, respectively. There was no statistically significant difference between CIS + DC and CSC–DC groups, but the two groups showed a significant decrease in MDR gene expression levels compared to CIS and DC groups. MDR gene expression was significantly downregulated (P < 0.001) in CSC–DC + CIS group as compared to CIS, DC, CIS + DC, CSC–DC, and (P < 0.01) CIS + DC groups (Fig. 6a).
Vaccination with CSC–DCs enhances the anti-tumor efficacy of cisplatin by downregulation of MDR and BCl-2 gene expression in tumor tissues of SEC-bearing mice. Data are the average fold change in a MDR and b Bcl-2 gene expression levels. Data are presented as the mean ± SD. #P < 0.05, *P < 0.01, **P < 0.001. a Significant decrease versus tumor control group, b Significant decrease versus CIS-treated group, c: Significant decrease versus DC-treated group, d Significant decrease versus CIS + DC-treated group, e Significant decrease versus CSC–DC-treated group. MDR: multi-drug resistance; Bcl-2: B-cell lymphoma 2; SEC: solid Ehrlich carcinoma, CIS: cisplatin; DC: dendritic cells, CSC–DC: CSC-pulsed dendritic cells; CIS + DC: combined treatment with CIS and DC; CIS + CSC–DC: combined treatment with CSC–DC and CIS
Bcl-2 relative gene expression was significantly downregulated in CIS, DC, CIS + DC, CSC–DC or CIS + CSC–DC groups (↓ 0.8 ± 0.04, ↓ 0.79 ± 0.03, ↓ 0.67 ± 0.03, ↓ 0.63 ± 0.06, and ↓ 0.24 ± 0.09, respectively) as compared with untreated control group, as shown in Fig. 6b. CIS + DC and CSC–DC groups significantly decreased in Bcl-2 gene expression levels compared to CIS (P < 0.05) and DC (P < 0.01) groups. Co-treatment with CIS and CSC-pulsed DCs significantly downregulated (P < 0.001) Bcl-2 gene expression compared to CIS, DC, CSC–DC, and (P < 0.01) CIS + DC groups (Fig. 6b).
Discussion
Recent research in CSCs’ biology is gradually gaining an increasing attention for developing potent and effective cancer therapies. This is due to CSCs high chemotherapeutic resistance which is responsible for treatment failure and subsequent tumor relapse [12, 22]. Therefore, it is essential to enhance the chemo-sensitivity of tumor cells via targeting drug-resistant CSCs. In the present study, we investigated the therapeutic efficiency of using enriched CSC-based DC vaccines either alone or in combination with low doses of cisplatin to overcome cisplatin resistance and inhibit tumorigenesis.
CSCs represent a small fraction in the tumor bulk comprising 1–2% of total tumor cells [23]. Since one of the defining characteristics of CSCs is their high chemotherapeutic drug resistance, we used chemotherapy drug selection method to enrich CSCs’ population in vitro in an EC cell line by applying different concentrations of CIS. As demonstrated in our study, treatment with CIS (50 ug/mL) was the concentration that showed the highest percentage of CSCs identified as CD44+/CD24− by flow cytometry and that was the highest concentration of cisplatin that cells could survive and grow.
Consistent with our findings, previous studies showed that selection based on chemotherapeutic drug resistance leads to an increase in the relative percentage of drug-resistant cell populations with stem cell characteristics [12, 19, 24, 25]. It was reported by Levina and his co-workers that drug treatment of lung cancer cells for 72 h successfully enriched stem cells population which is in line with our findings [26].
Moreover, previous study of Goldman et al. demonstrated that the exposure of human breast cancer cells to high doses of taxanes in vitro induced changes in the cells population phenotype towards CD44hiCD24hi [27]. Another study demonstrated that the exposure of hepatocellular carcinoma non-CSCs to carboplatin induced their phenotypic transition into cells with stem-like properties and high tumorigenic capacity which supports our findings [28].
In the current study, we prepared enriched CSCs lysate-based DC vaccine in vitro with the aim of inducing anti-tumor immune response against antigens loaded on the DCs. Our findings demonstrated that the antigen loading of DCs with the enriched CSCs lysate reserved the phenotypical characteristics of the generated mature DCs as shown by the non-significant difference between loaded or unloaded DCs in CD11c, CD83, and CD86 which is in line with the work of Xu et al. [29].
The effectiveness of chemotherapies is limited by the acquisition of MDR of tumor cells which is often mediated by CSCs [30, 31]. Accumulating evidence indicate that chemotherapy-resistant stem cells upregulates MDR gene expression, thereby reducing the efficacy of these drugs [32, 33]. Alisi et al. demonstrated that upregulation of MDR is probably one of the important mechanisms of CSCs in response to chemotherapy due to their increased drug efflux capacity [30].
Our findings showed upregulation of MDR gene expression in untreated control group compared to treated groups. In contrast, the co-treated group with CSC–DC and repetitive low doses of CIS showed anti-tumor chemo-sensitizing effects mediated by significant downregulation of MDR relative gene expression levels compared to all other treated groups [34]. This indicates MDR-reversing effect of this treatment strategy via dual targeting of both resistant CSCs and non-CSCs.
Vaccination with CSC–DC showed a profound tumor growth inhibition when combined with CIS as demonstrated by our results where the co-treated group showed a significant tumor growth inhibition by 80% compared to either single treatment with CSC–DC (66.7%) or CIS (41%). The administration of CSC–DC either alone or in combination showed a better survival. In line with our results, a previous study showed the therapeutic efficacy of using CSC–DC-based vaccine in inhibiting tumor growth and recurrence as well as prolonging the survival of tumor-bearing animals [35].
It has been reported that the development of malignancy is associated with upregulation of apoptosis inhibitory genes such as Bcl-2 and the production of cells with MDR phenotype [36]. MDR property of CSCs is associated with modulation of apoptotic pathways. Dysregulated apoptotic signaling pathways in CSCs make them able to escape apoptosis by altering Bcl-2 expression levels and, therefore, maintain tumor growth which represents another mechanism for chemo-resistance. This leads to the failure of chemotherapies, rapid tumor progression, and metastasis [4, 12].
In the present study, we demonstrated a significant decrease in Bcl-2 relative gene expression in all treated groups compared to control untreated group. In addition, CSC-pulsed DCs showed a significant decrease in Bcl-2 relative gene expression when compared to DCs. Moreover, we found a statistically significant decrease in Bcl-2 relative gene expression level in CSC–DC + CIS group as compared to single treatment with either CSC–DC vaccine or CIS. These findings suggest that co-treatment with CSC–DC vaccine leads to restoration of apoptotic pathways through downregulation of Bcl-2 gene expression. These results are consistent with the histopathological observations that showed an increase in the apoptotic figures in CSC–DC + CIS co-treated group. This is consistent with the previous study that shows that DCs pulsed with CSC antigens might have unique therapeutic benefits where they induce a significant increase in apoptosis compared to unloaded DCs [37].
Taken together, the present study suggests that CSC–DC vaccine significantly increased the efficacy of CIS by increasing tumor cells sensitivity to CIS which is likely due to decreased MDR and Bcl-2 gene expression levels achieved by dual targeting of resistant cells with CSCs phenotype and non-CSCs. In line with our findings, it was previously reported that enriched CSC-based vaccines significantly decrease tumor volume, prolong survival, inhibit of metastasis, and induces humoral immune responses against cancer stem cells [38].
Our results suggested that the generation of CSCs based on their chemotherapeutic drug resistance property may be helpful in developing new immunotherapeutic strategies which could increase the efficacy of conventional chemotherapies. The main limitations of this study are that we were not able to confirm whether the CD44+/CD24− drug-resistant cell population was actually CSCs or they were expressing CSC-like phenotype. Moreover, the genetic diversity of CSCs in different types of cancer is another limitation of our study, since we studied only one cell line (EC) [39].
One of the reasons of cancer immunotherapy failure is the immunosuppressive tumor microenvironment. Eliminating tumor suppressive immune networks can lead to improvement in cancer immunotherapies such as DC-based vaccine [40]. In addition, the administration of conventional chemotherapies, especially at high doses—that target malignant cells—is associated with toxic effects as well as induction of immune suppression.
It has been reported that repetitive administration of low-dose chemotherapy (known as metronomic chemotherapy) selectively eliminates some of the immunosuppressive networks via depleting regulatory T cells and preconditions the tumor microenvironment for immunotherapy by increasing the tumor permeability to CD8 T-cell-derived cytolytic factors which eventually result in an improvement in immunotherapy efficacy along with the restoration of anti-tumor immune responses in the absence of toxicity [1, 15, 36]. Several studies showed improvement in the immune responses following low doses of chemotherapeutic agents which supports our findings [41,42,43,44,45].
Conclusion
Elimination of CSCs represents an attractive treatment strategy to overcome chemo-resistance and prevent tumor relapse. Since CSCs represents a minor population within the tumor mass, it is likely that combinatorial therapy that targets both drug-resistant CSCs and conventional chemotherapy that abolishes the proliferating cells of the tumor bulk might have a better therapeutic effect with success in clinical trials.
Chemo-resistance to cisplatin (MDR gene expression) could be reduced in SEC-bearing mice upon targeting CSC by a prepared CSC–DC-based vaccine. Dual targeting of CSCs and non-CSCs suppressed tumor growth and induced apoptosis. These results were confirmed by histopathological examination which showed enhanced apoptosis with the absence of mitotic figures. It is foreseeable that this therapeutic combination will be further studied in other tumor models to exploit the underlying mechanisms governing CSCs and to enhance the efficacy of the currently used chemotherapies.
References
Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, Lee YK, Kwon HY. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;28:5416923. https://doi.org/10.1155/2018/5416923.eCollection.
Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25(1):20. https://doi.org/10.1186/s12929-018-0426-4.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60.
Salem ML, El-Badawy AS, Li Z. Immunobiology and signaling pathways of cancer stem cells: implication for cancer therapy. Cytotechnology. 2015;67(5):749–59. https://doi.org/10.1007/s10616-014-9830-0.
Sastry KS, Al-Muftah MA, Li P, Al-Kowari MK, Wang E, Ismail Chouchane A, Kizhakayil D, Kulik G, Marincola FM, Haoudi A, Chouchane L. Targeting proapoptotic protein BAD inhibits survival and self-renewal of cancer stem cells. Cell Death Differ. 2014;21(12):1936–49. https://doi.org/10.1038/cdd.2014.140.
Goltsev AN, Babenko NN, Gaevskaya YA, Chelombitko OV, Dubrava TG, Bondarovich NA, Ostankov MV, Klochkov VK, Kavok NS, Malyukin Y. Application of nanoparticles based on rare earth orthovanadates to inactivate ehrlich carcinoma growth. Biotechnologia Acta. 2015;8(4):113–21.
Goltsev AN, Babenko NN, Gaevskaya YA, Bondarovich NA, Dubrava TG, Ostankov MV, Chelombitko OV, Malyukin YV, Klochkov VK, Kavok NS. Nanotechniques inactivate cancer stem cells. Nanoscale Res Lett. 2017;12(1):415. https://doi.org/10.1186/s11671-017-2175-9.
Roh JL, Kim EH, Park JY, Kim JW. Inhibition of glucosylceramide synthase sensitizes head and neck cancer to cisplatin. Mol Cancer Ther. 2015;14:1907–15.
Cataldo A, Cheung DG, Balsari A, Tagliabue E, Coppola V, Iorio MV, Palmieri D, Croce CM. miR-302b enhances breast cancer cell sensitivity to cisplatin by regulating E2F1 and the cellular DNA damage response. Oncotarget. 2016;7:786–97. https://doi.org/10.18632/oncotarget.
Tao K, Yin Y, Shen Q, Chen Y, Li R, Chang W, Bai J, Liu W, Shi L, Zhang P. Akt inhibitor MK-2206 enhances the effect of cisplatin in gastric cancer cells. Biomed Rep. 2016;4:365–8.
Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular Mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3(1):1351–71. https://doi.org/10.3390/cancers3011351.
Xie Q, Wang S, Zhao Y, Zhang Z, Qin C, Yang X. MiR-519d impedes cisplatin-resistance in breast cancer stem cells by down-regulating the expression of MCL-1. Oncotarget. 2017;8(13):22003–13. https://doi.org/10.18632/oncotarget.15781.
Salem ML. The use of dendritic cells for peptide-based vaccination in cancer immunotherapy. Methods Mol Biol. 2014;1139:479–503. https://doi.org/10.1007/978-1-4939-0345-0_37.
El-Ashmawy NE, Khedr NF, El-Bahrawy HA, Abo Mansour HE. Metformin augments doxorubicin cytotoxicity in mammary carcinoma through activation of adenosine monophosphate protein kinase pathway. Tumor Biol. 2017;39(5):1010428317692235. https://doi.org/10.1177/1010428317692235.
Salem ML, Abdel Salam SG, Nassef M, Hammad S, El Adl R. Immunoenhancing properties of the anti-tumor effects of adoptively transferred T cells with chemotherapeutic cyclophosphamide by co-administration of bone marrow cells. J Basic Appl Zool. 2015;72:96–103.
Shahabuddin MS, Nambiar M, Moorthy BT, Naik PL, Choudhary B, Advirao GM, Raghavan SC. A novel structural derivative of natural alkaloid ellipticine, MDPSQ, induces necrosis in leukemic cells. Invest New Drugs. 2011;29(4):523–33. https://doi.org/10.1007/s10637-009-9379-5.
Xu K, Shen K, Liang X, Li Y, Nagao N, Li J, Liu J, Yin P. MiR-139-5p reverses CD44+/CD133+ associated multidrug resistance by downregulating NOTCH1in colorectal carcinoma cells. Oncotarget. 2016;7(46):75118–29. https://doi.org/10.18632/oncotarget.12611.
Salem ML, Eissa IR, Mohamed TM. Dendritic cells generated from naïve and tumor-bearing mice uniquely restores different leukocyte subpopulations in chemotherapy-treated tumor-bearing mice. Clin Cancer Investig J. 2016;5:1–10.
El-Ashmawy NE, El-Zamarany EA, Salem ML, Khedr EG, Ibrahim AO. A new strategy for enhancing antitumor immune response using dendritic cells loaded with chemo-resistant cancer stem-like cells in experimental mice model. Mol Immunol. 2019;111:106–17. https://doi.org/10.1016/j.molimm.2019.04.001(Epub 2019 Apr 30).
Li Q, Lu L, Tao H, Xue C, Teitz-Tennenbaum S, Owen JH, Moyer JS, Prince ME, Chang AE, Wicha MS. Generation of a novel dendritic-cell vaccine using melanoma and squamous cancer stem cells. J Vis Exp. 2014;83:e50561. https://doi.org/10.3791/50561.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)). Methods. 2001;25(4):402–8.
Agliano A, Calvo A, Box C. The challenge of targeting cancer stem cells to halt metastasis. Semin Cancer Biol. 2017;44:25–42. https://doi.org/10.1016/j.semcancer.2017.03.003.
Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, Fearon ER, Lawrence TS, Xu L. MicroRNA miR inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE. 2009;4(8):6816. https://doi.org/10.1371/journal.pone.0006816.
Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L, Ambudkar SV. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst. 2010;102(21):1637–52. https://doi.org/10.1093/jnci/djq361.
Wan F, Zhang S, Xie R, Gao B, Campos B, Herold-Mende C, Lei T. The utility and limitations of neurosphere assay, CD133 immunophenotyping and side population assay in glioma stem cell research. Brain Pathol. 2010;20(5):877–89. https://doi.org/10.1111/j.1750-3639.2010.00379.x.
Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS ONE. 2008;3(8):e3077.
Goldman A, Majumder B, Dhawan A, Ravi S, Goldman D, Kohandel M, Majumder PK, Sengupta S. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy induced phenotypic transition. Nat Commun. 2015;11:6139.
Hu X, Ghisolfi L, Keates AC, Zhang J, Xiang S, Lee DK, Li CJ. Induction of cancer cell stemness by chemotherapy. Cell Cycle. 2012;11:2691–8.
Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27(8):1734–40. https://doi.org/10.1002/stem.102.
Alisi A, Cho WC, Locatelli F, Fruci D. Multi drug resistance and cancer stem cells in neuroblastoma and hepatoblastoma. Int J Mol Sci. 2013;14(12):24706–25. https://doi.org/10.3390/ijms141224706.
Doherty MR, Smigiel JM, Junk DJ, Jackson MW. Cancer stem cell plasticity drives therapeutic resistance. Cancers (Basel). 2016;8(1):pii: E8. https://doi.org/10.3390/cancers8010008.
Safa AR. Resistance to cell death and its modulation in cancer stem cells. Crit Rev Oncog. 2016;21(3–4):203–19. https://doi.org/10.1615/CritRevOncog.2016016976.
Salaroglio IC, Panada E, Moiso E, et al. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol Cancer. 2017;16:91. https://doi.org/10.1186/s12943-017-0657-0.
Shafiei-Irannejad V, Samadi N, Yousefi B, Salehi R, Velaei K, Zarghami N. Metformin enhances doxorubicin sensitivity via inhibition of doxorubicin efflux in P-gp-overexpressing MCF-7 cells. Chem Biol Drug Des. 2018;91(1):269–76. https://doi.org/10.1111/cbdd.13078.
Hu Y, Lu L, Xia Y, Chen X, Chang AE, Hollingsworth RE, Hurt E, Owen J, Moyer JS, Prince ME, Dai F, Bao Y, Wang Y, Whitfield J, Xia JC, Huang S, Wicha MS, Li Q. Therapeutic efficacy of cancer stem cell vaccines in the adjuvant setting. Cancer Res. 2016;76(16):4661–72. https://doi.org/10.1158/0008-5472.CAN-15-2664Epub 2016 Jun 20.
Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26(2):263–70.
Jiang T, Chen X, Zhou W, Fan G, Zhao P, Ren S, Zhou C, Zhang J. Immunotherapy with dendritic cells modified with tumor-associated antigen gene demonstrates enhanced antitumor effect against lung cancer. Transl Oncol. 2017;10(2):132–41. https://doi.org/10.1016/j.tranon.2016.12.002Epub 2017 Jan 25.
Lu L, Tao H, Chang AE, Hu Y, Shu G, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology. 2015;4:e990767.
Xie X, Teknos TN, Pan Q. Are all cancer stem cells created equal? Stem Cells Transl Med. 2014;3(10):1111–5. https://doi.org/10.5966/sctm.2014-0085.
Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Rea D, Barbieri A, Arra C, Maiolino P, Tornesello M, Ciliberto G, Buonaguro FM, Buonaguro L. A novel multidrug metronomic chemotherapy significantly delays tumor growth in mice. J Transl Med. 2016;14:58. https://doi.org/10.1186/s12967-016-0812-1.
Ehrke MJ. Immunomodulation in cancer therapeutics. Int Immunopharmacol. 2003;3:1105–19.
Mihich E. Anticancer drug-induced immunomodulation and cancer therapeutics. Curr Cancer Ther Rev. 2007;3:174–93.
Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–87.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73.
Zhang T, Herlyn D. Combination of active specific immunotherapy or adoptive antibody or lymphocyte immunotherapy with chemotherapy in the treatment of cancer. Cancer Immunol Immunother CII. 2009;58(4):475–92. https://doi.org/10.1007/s00262-008-0598-y.
Acknowledgements
The authors are grateful to Dr. Noha El-Anwar, Lecturer of Pathology, Faculty of Medicine, Tanta University, Egypt for her assistance in the histopathological examination of the tumor sections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Ashmawy, N.E., Salem, M.L., Khedr, E.G. et al. Dual-targeted therapeutic strategy combining CSC–DC-based vaccine and cisplatin overcomes chemo-resistance in experimental mice model. Clin Transl Oncol 22, 1155–1165 (2020). https://doi.org/10.1007/s12094-019-02242-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02242-4