Abstract
Purpose
Capecitabine has been studied as a radiosensitizer, and our study seeks to examine the association of concurrent capecitabine/radiation therapy (RT) on event-free- (EFS) and overall survival (OS) in women with breast cancer (BC) with residual disease after neoadjuvant chemotherapy (NAC).
Methods/patients
In a retrospective study of women with BC who received adriamycin/taxane-based NAC from 2004–2016, we identified 21 women administered concurrent capecitabine/RT. To assess differences in survival, we selected a clinical control cohort (n = 57) based on criteria used to select patients for capecitabine/RT. We also created a matched cohort (2:1), matching on tumor subtype, pathological stage and age (< 50 or 50+ years). Differences in EFS, using STEEP criteria, and OS, using all-cause mortality, between those who received capecitabine/RT and controls were assessed.
Results
Of the 21 women who received capecitabine/RT, median age was 52 years. The majority were pathologic stage III (n = 15) and hormone receptor-positive/HER2-negative BC (n = 20). In those receiving capecitabine/RT, there were 9 events, compared with 14 events in clinical and 10 events in matched controls. Capecitabine/RT was associated with worse OS in clinical (HR 3.83 95% CI 1.12–13.11, p = 0.03) and matched controls (HR 3.71 95% CI 1.04–13.18, p = 0.04), after adjusting for clinical size, pathological stage and lymphovascular invasion. Capecitabine/RT was also associated with a trend towards worse EFS in clinical (HR 2.41 95% CI 0.86–6.74, p = 0.09) and matched controls (HR 2.68 95% CI 0.91–7.90, p = 0.07) after adjustment.
Conclusion
Concurrent capecitabine/RT after NAC is associated with worse survival and should be carefully considered in BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among women with locally advanced breast cancer (BC), residual disease after neoadjuvant chemotherapy (NAC) predicts survival, and a higher burden of disease is associated with increased recurrences and a poor prognosis [1]. Current management is multidisciplinary and involves chemotherapy followed by radiation therapy (RT) [2].
Capecitabine is an oral prodrug of fluorouracil which interferes with DNA synthesis[3] and is well tolerated [4]. In BC, capecitabine has been shown to stabilize disease and prevent progression in women with metastatic BC after failing anthracycline therapy [5, 6]. However, in an elderly population, adjuvant capecitabine was found to be inferior to standard chemotherapy [7]. Its role in conjunction with traditional adjuvant therapies in early BC is being investigated, and a recent meta-analysis found that adjuvant capecitabine may improve overall survival (OS) but not disease-free survival, with its utility varying by tumor subtype and disease burden [8]. However, studies in the neoadjuvant setting in those with residual disease are limited.
Radiation is an important adjuvant therapy and has been shown to reduce the rates of locoregional recurrence and improve disease-free survival and overall mortality [9]. Although traditionally administered after adjuvant chemotherapy, concurrent chemoradiotherapy is being explored in locally advanced BC [10]. Capecitabine has been extensively studied as a radiosensitizer in rectal cancer and has been shown to decrease recurrences and improve cancer-related survival with no additional complications when administered concomitantly [11, 12]. In pancreatic cancer, capecitabine as a radiosensitizer shows comparable OS as historical controls in the adjuvant setting [13], and meta-analyses suggest that it is as efficacious as gemcitabine/RT in locally advanced pancreatic cancer [14].
In BC, some studies suggest that concomitant chemoradiotherapy may offer better locoregional control as compared with chemotherapy and subsequent radiation in node-positive BC after surgery [15]. However, the role of capecitabine as a radiosensitizer in conjunction with RT in those with residual BC after modern NAC is unknown. Our study seeks to examine the association of concurrent capecitabine/RT on event-free and OS in women with BC with residual disease after NAC.
Materials and methods
Patient population: concurrent capecitabine/RT and clinical controls
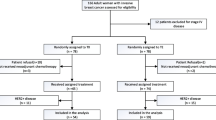
Retrospective review identified 317 unique women who were diagnosed with BC between January 2004 and February 2016, administered NAC, underwent surgery, and received part of their care at Columbia University Medical Center (CUMC). We excluded n = 6 women who received non-adriamycin/taxane (A/T)-based NAC (all received CMF), n = 1 who was pregnant and did not receive NAC, and n = 3 women with an unknown NAC regimen, leaving 307 women who were administered A/T-based NAC. We excluded n = 31 who never received RT and n = 1, who initially received capecitabine concurrently with RT but then received treatment dose capecitabine for a planned 6-month course, leaving a cohort of 275 women. As the first patient who received capecitabine/RT in this cohort was in 2010, we limited our population to those diagnosed between 2010 and 2016 (excluding n = 61), resulting in a total of n = 214 women. As capecitabine/RT was only given to those with high risk but not metastatic disease after NAC, we excluded those women who achieved a pathologic complete response (pCR) (n = 61), had pathologic stage I disease (n = 48), or had metastatic disease (n = 5). Finally, we excluded the n = 3 women who were diagnosed with inflammatory BC and the n = 19 who had HER2-positive disease, resulting in a cohort of n = 78 women eligible for concurrent capecitabine/RT. Of these, n = 21 received concurrent capecitabine with RT, leaving n = 57 women to serve as clinical controls. All research was conducted in accordance with CUMC IRB approved protocol (IRB # AAAJ8512).
Matched controls
To ensure that the controls were comparable to those treated with capecitabine/RT, the 21 women who received capecitabine/RT were matched in a 2:1 fashion based on tumor subtype (hormone receptor +/HER2-negative or triple-negative breast cancer, TNBC), pathological stage, and age (< 50 and 50+ years). No patients had HER2+ breast cancer. Matches were drawn from the remaining n = 254 women who did not receive capecitabine/RT but who received A/T-based NAC and RT to allow for optimal 2:1 matching. Of the matches, n = 31 of the women were also amongst the clinical controls.
Clinical and pathological variables
Clinicopathologic data were abstracted from medical records by three independent researchers. All data were double-verified, and discrepancies were resolved by oncologists EC and KK. Age was defined in years at BC diagnosis and was stratified into < 50 years of age and ≥ 50 years of age. Race/ethnicity was categorized as non-hispanic white, non-hispanic black, hispanic and asian/other based on self-report or physician notes. Tumor size was defined as the largest dimension on any imaging modality prior to any treatment and was analyzed as a continuous variable and stratified at 0–5 cm and > 5 cm. Grade was defined as the highest grade seen on any biopsy. Estrogen receptor (ER) and progesterone receptor (PR) positivity was defined as 1% or greater expression on any biopsy in accordance with American Society of Clinical Oncology/College of American Pathologist (ASCO/CAP) guidelines from 2010 [16]. Tumors were considered HER2 positive if they were 3+ by immunohistochemistry (IHC), demonstrated gene amplification with a ratio of Her-2/CEP17 ≥ 2.2 by in situ hybridization or had HER2 average copies/cell ≥ 6 on either the core biopsy or surgical pathology specimen [17]. Based on prior studies, subtype groups were defined as (a) hormone receptor positive (HR+: ER and/or PR positive) and HER2 negative, (b) HER2 positive regardless of hormonal status, and (c) TNBC (ER, PR, and HER2 negative) [18]. Clinical and pathological staging were determined based on the American Joint Committee on Cancer (AJCC) TNM Staging Manual, 7th edition [19]. Pathologic complete response (pCR) was defined as no residual invasive disease in the breast or lymph nodes on surgical pathology specimens (ypT0/Tis ypN0). Presence of lymphovascular invasion (LVI) was assessed on surgical pathology specimens and defined based on the CUMC standard pathological definition as presence of carcinoma cells within a definite endothelial-lined space (either lymphatic or blood vessels). This was rarely verified using D2-40 immuno-histochemical stain for lymphatic endothelium and CD31 for endothelium of all vessels.
All women received A-based, T-based, or A/T-based NAC. Women were considered to have received RT if they received any type of whole breast radiation with or without nodal radiation. Dose of capecitabine administered with RT was chosen at the discretion of the medical oncologist and ranged from 1000 to 2000 mg per day (Monday–Friday) given in two divided doses over the 6–8 weeks of RT. Hormonal therapy was defined as treatment with tamoxifen only or any aromatase inhibitor (AI). Surgery type was stratified into lumpectomy or mastectomy with or without lymph node dissection. As both capecitabine and RT have been shown to induce lymphopenia [4, 20,21,22], absolute lymphocyte count prior to surgery and after radiation (within 3 months) were abstracted.
Statistical analysis
Chi-square, Fisher’s exact, and t tests were used to compare relevant clinical and pathological variables at baseline according to administration of concurrent capecitabine/RT. Event-free survival (EFS) was based on the STEEP criteria[23], and events were defined as any local/regional or distant metastasis, contralateral invasive BC (excluding in situ disease), any secondary, non-breast, invasive cancer, and/or death by any cause. EFS and OS were calculated in months from date of diagnosis to date of first event or death (for OS) or last follow-up in those without events. Kaplan–Meier survival analysis and the log-rank statistic were used to evaluate survival differences between those who received concurrent capecitabine/RT compared with clinical and matched controls. Cox proportional hazard models were used to estimate the univariate hazard ratios for EFS and OS and to assess the association of capecitabine/RT and EFS and OS after adjusting for relevant clinical covariates. If a difference in survival was detected, further analyses would be conducted to help elucidate a potential mechanism. All analyses were performed using SAS 9.4 and STATA 12.0 with significance defined as a two-sided p value of less than or equal to 0.05. For the matched controls, frailty models were also performed to account for the correlation between the matched pairs.
Results
Patient demographics
Of the 78 women, 21 (27%) received capecitabine with radiation. Among these, median age was 52 years [mean 51.5, standard deviation (SD) 13.5 years], and the majority were non-hispanic white (n = 7) or hispanic (n = 10) and had pathologic stage III disease (n = 15). The majority (n = 20) had HR+/HER2− subtype, and n = 1 had TNBC, Table 1.
Compared with clinical controls, those who received capecitabine/RT were more likely to have LVI (75 vs. 46%, p = 0.03) and have a trend towards larger tumors (43 vs. 23% with tumor size > 5 cm, p = 0.08) and higher rate of mastectomy (90 vs. 74%, p = 0.13). Compared to matched controls, those who received capecitabine/RT were more likely to have LVI (75 vs. 46%, p = 0.04) and to have larger tumors (43 vs. 19% with tumors > 5 cm in size, p = 0.05). Although not significantly different, those who received capecitabine/RT were more likely to be pathologic stage IIIC as compared to clinical and matched controls. There were no differences in age, race, grade, tumor subtype, or hormonal therapy use between those who received capecitabine/RT and either control group (p > 0.05), Table 1.
Among those who received concurrent capecitabine/RT, n = 16 women who had HR+/HER2− BC received hormonal therapy. Of the remaining four women with HR+/HER2− BC, n = 2 never received hormonal therapy as they had disease progression, and n = 2 had missing data. For both the clinical and matched controls, n = 1 woman in each group had HR+/HER2− BC and refused hormonal therapy, but all other women received hormonal therapy. In addition, all women received RT, and there were no significant differences between breast only and breast plus nodal RT between the groups (p > 0.05) as all the women who received concurrent capecitabine/RT received breast plus nodal RT (one had missing data), and only three women in the clinical and two women in the matched controls received breast only RT.
Survival analysis
In those receiving capecitabine/RT, there were nine events (three local recurrences, six distant recurrences) and eight deaths. There were 14 events (4 local recurrences, 9 distant recurrences, 1 death without recurrence) and 7 deaths in the clinical controls, and 10 events (9 distant recurrences, 1 death without recurrence) and 5 deaths in the matched controls.
Clinical control analysis
Compared with clinical controls, concurrent administration of capecitabine/RT was associated with worse EFS (HR 2.95 95% CI 1.25–6.93, p = 0.01) and OS (HR 5.23 95% CI 1.84–14.83 p < 0.01) on univariate analysis. In multivariate models, capecitabine/RT was associated with worse OS (HR 3.83 95% CI 1.12–13.11, p = 0.03), after adjusting for clinical size (continuous), pathological stage, and LVI. The association with EFS was attenuated (HR 2.41 95% CI 0.86–6.74, p = 0.09), but the effect size was similar, Table 2/Fig. 1. For multivariate models, pathological stage was collapsed into three categories (IIA/IIB, IIIA/IIIB, and IIIC) due to low numbers and events in the IIB and IIIB groups.
Matched analysis
In the 2:1 matched cohort, all women were matched successfully by subtype (HR+/HER2− or TNBC). All, but two women were matched by pathological staging, as one woman with stage IIIC disease was matched with one woman with stage IIIB disease and another with stage IIIC disease was matched with two women who were stage IIIB and IIIA, respectively. All but three women were successfully matched by age group, and the average difference in age was 10 years among those three pairs. To maximize matching, two of the matches had inflammatory BC. Compared with matched controls, capecitabine/RT was associated with worse EFS (HR 3.11 95% CI 1.24–7.83 p = 0.02) and OS (HR 5.44 95% CI 1.71–17.35 p < 0.01) on univariate analysis. In multivariate models, capecitabine/RT was associated with worse OS (HR 3.71 95% CI 1.04–13.18 p = 0.04), after adjusting for clinical size, pathological stage, and LVI. The association with EFS was attenuated (HR 2.68 95% CI 0.91–7.90 p = 0.07), but the effect size was again similar, Table 3/Fig. 2.
Lymphocyte count
Absolute lymphocyte count (ALC) fell on average 0.46 points (SD 0.72) between levels checked prior to breast surgery and within 3 months of RT in 16 women who received concurrent capecitabine/RT with available ALC data, and 4 women (24%) had grade 3–4 lymphopenia. In matched controls, levels fell on average 0.03 points (SD 0.55) in the 20 women with available data, with 1 woman (5%) having grade 3–4 lymphopenia. This was significantly less than in those receiving concurrent capecitabine/RT, p = 0.05. However, in clinical controls, levels fell on average 0.24 points (SD 0.54) in the 22 women with available date within this same timeframe, which was not significantly different, p = 0.29. Overall, ten of the women had ALC levels checked before the end of RT, but all were within 6 weeks of the end of RT (mean 4 weeks, range 2–6 weeks).
Discussion
Women with invasive BC receiving concurrent capecitabine/RT after NAC had more LVI and larger tumors as compared to controls but were otherwise similar. Compared with both control groups, women receiving concurrent capecitabine/RT experienced worse survival, even after adjusting for clinical size, pathological stage and LVI.
Although the role of capecitabine in metastatic BC is well established, its role as adjuvant chemotherapy, particularly combined with adjuvant RT for BC, is still unclear. The clinical FinXX trial showed no survival benefit in those administered capecitabine in addition to cyclophosphamide, docetaxel and epirubicin as adjuvant therapy in women with early BC. There was a suggestion of favorable survival outcomes in the subgroup of TNBC, but the study excluded those who received NAC [24]. Martin et al. studied capecitabine with epirubicin plus docetaxel (ET-X as compared to epirubicin plus cyclophosphamide followed by docetaxel (EC-T) as adjuvant therapy in women with operable node-positive BC and found that EC-T conferred superior OS but not disease-free survival [25]. In the neoadjuvant setting, the CREATE-X study examined capecitabine (1250 mg/m2 twice daily over 6 months) plus standard adjuvant therapy vs. standard therapy alone in women with residual disease after modern A/T-based NAC and found that the addition of capecitabine significantly improved disease-free and OS [26]. Finally, a meta-analysis by Zhang et al. found that capecitabine, in doses ranging from 1600 to 2500 mg/m2 daily, with standard adjuvant regimens in early BC improved OS but only improved disease-free survival in certain subtypes (TNBC) and in those with high-risk features (lymph node involvement and high Ki67) [8].
Additionally, few have investigated concomitant administration of capecitabine with RT for BC, and no one has examined this in the neoadjuvant setting. Rouesse et al. compared 5-fluorouracil, mitoxantrone and cyclophosphamide with concomitant radiotherapy vs. 5-fluorouracil, epirubicin, and cyclophosphamide with subsequent radiotherapy and found that concomitant chemoradiotherapy was associated with significantly better locoregional control in node-positive BC after surgery, but they found slightly more acute toxicity [15]. Shaughnessy et al. studied twenty patients with recurrent or advanced BC that was considered inoperable who received concurrent radiotherapy and capecitabine, paclitaxel or cisplatin/etoposide and found durable local disease control and acceptable toxicities, but there was no control group to assess survival differences [27].
Our study investigated the role of capecitabine, in much lower doses than traditional adjuvant chemotherapy, administered concurrently with RT as a radiosensitizer in women with residual disease after modern A/T-based NAC. We found that concomitant capecitabine/RT was associated with worse outcomes as compared to two control groups, even after adjusting for other poor prognostic factors. Those who received capecitabine/RT were more likely to be pathologic stage IIIC and may have had more residual disease burden after NAC, although surgical size and number of positive lymph nodes were not significantly different as compared to either control group, p > 0.05. This suggests that supraclavicular or internal mammary lymph node involvement may have been more critical than number of positive lymph nodes and residual tumor size. Upon detailed review, seven of the twenty-one women experienced disease progression during NAC and went to surgery prior to completing NAC. They were also more likely to have LVI, which we previously reported to be independently associated with worse survival in those receiving NAC [28]. However, adjusting for these differences in multivariate models only attenuated the association, suggesting that although women receiving concurrent capecitabine/RT may have a higher pathological stage and more LVI, these factors did not account for all of the negative impacts on survival. Of note, all but one of the women administered capecitabine in our study was HR+/HER2−, and the suggestion of benefit in prior trials was more pronounced in TNBC [24, 26].
The mechanism by which concurrent capecitabine/RT may contribute to worse outcomes is unknown. RT has been shown to cause immunosuppression by lowering lymphocyte counts and increasing infection [20,21,22, 29] and some studies suggest that concurrent chemoradiotherapy may be associated with enhanced late toxicities [30]. Capecitabine has also been shown to reduce lymphocyte count[6], and lymphopenia has been shown to predict chemotherapy toxicity as well as poorer OS in BC, sarcoma and lymphomas [31]. We found that ALC fell to a larger degree in women receiving concurrent capecitabine/RT as compared with controls after RT although our results are limited by significant missing data in controls. This may be dose-dependent, and of the 16 women with detailed capecitabine dosing, 5 received 1000 mg daily, 3 received 1500 mg daily, and 8 received 2000 mg daily. Of those who received the highest dose (2000 mg daily), 3 had grade 3 lymphopenia; whereas, in those women receiving 1000–1500 mg daily, only 1 had grade 3 lymphopenia. While this is hypothesis generating, concurrent capecitabine/RT may depress ALC leading to immunosuppression and worse outcomes. In addition, concurrent administration of capecitabine with RT did not compromise ability to deliver RT as all women were able to complete their planned course of radiation in a timely manner, with no significant increase in acute radiation toxicity.
Strengths of this study include a diverse population with assessment of multiple clinicopathologic variables as well as the use of two control populations. Although not statistically significant, there were a higher proportion of black and hispanic women in the capecitabine/RT group as compared with controls, suggesting a more aggressive phenotype [32] resulting in more aggressive multi-modality treatments. Limitations include small sample size, incomplete assessment of lymphocyte count in all patients, and the retrospective nature of our study owing to potential for residual confounding. As our study is descriptive and reports on a limited number of cases, our results should be considered hypothesis generating. Future studies should also investigate the role of immunosuppression.
Conclusion
Administration of concurrent capecitabine/RT may confer worse survival in those with residual disease after NAC as compared to controls, and the use of capecitabine as a radiosensitizer in BC should be carefully considered.
Abbreviations
- NAC:
-
Neoadjuvant chemotherapy
- RT:
-
Radiation therapy
- BC:
-
Breast cancer
- TNBC:
-
Triple-negative breast cancer
- pCR:
-
Pathological complete response
- EFS:
-
Event-free survival
- OS:
-
Overall survival
References
Chaudry M, Lei X, Gonzalez-Angulo AM, et al. Recurrence and survival among breast cancer patients achieving a pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2015;153(2):417–23.
Rustogi A, Budrukkar A, Dinshaw K, Jalali R. Management of locally advanced breast cancer: evolution and current practice. J Cancer Res Ther. 2005;1(1):21–30.
Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34(8):1274–81.
Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23–44.
Blum JL. Xeloda in the treatment of metastatic breast cancer. Oncology. 1999;57(Suppl 1):16–20.
Wagstaff AJ, Ibbotson T, Goa KL. Capecitabine: a review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer. Drugs. 2003;63(2):217–36.
Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–65.
Zhang ZC, Xu QN, Lin SL, Li XY. Capecitabine in combination with standard (neo) adjuvant regimens in early breast cancer: survival outcome from a meta-analysis of randomized controlled trials. PLoS One. 2016;11(10):e0164663.
McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35.
Mandilaras V, Bouganim N, Spayne J, et al. Concurrent chemoradiotherapy for locally advanced breast cancer-time for a new paradigm? Curr Oncol (Toronto, Ont). 2015;22(1):25–32.
Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324(11):709–15.
Zhu AX, Willett CG. Chemotherapeutic and biologic agents as radiosensitizers in rectal cancer. Semin Radiat Oncol. 2003;13(4):454–68.
Abrams MJ, Huber KE, Knisely JP, Chang BW, Russo SM, Saif MW. Capecitabine as a radiosensitizer in adjuvant chemoradiotherapy for pancreatic cancer: a retrospective study. Anticancer Res. 2015;35(12):6901–7.
Yang YF, Cao XH, Bao CE, Wan X. Concurrent radiotherapy with oral fluoropyrimidine versus gemcitabine in locally advanced pancreatic cancer: a systematic review and meta-analysis. OncoTargets Ther. 2015;8:3315–22.
Rouesse J, de la Lande B, Bertheault-Cvitkovic F, et al. A phase III randomized trial comparing adjuvant concomitant chemoradiotherapy versus standard adjuvant chemotherapy followed by radiotherapy in operable node-positive breast cancer: final results. Int J Radiat Oncol Biol Phys. 2006;64(4):1072–80.
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract Am Soc Clin Oncol. 2010;6(4):195–7.
Tchrakian N, Flanagan L, Harford J, Gannon JM, Quinn CM. New ASCO/CAP guideline recommendations for HER2 testing increase the proportion of reflex in situ hybridization tests and of HER2 positive breast cancers. Virchows Arch. 2016;468(2):207–11.
Zhang C, Wang S, Israel HP, et al. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. SpringerPlus. 2015;4:386.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–80.
Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62(5):1423–6.
Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94(3):571–9.
Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–32.
Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Adjuvant capecitabine in combination with docetaxel, epirubicin, and cyclophosphamide for early breast cancer: the randomized clinical FinXX trial. JAMA Oncol. 2017;3(6):793–800.
Martin M, Ruiz Simon A, Ruiz Borrego M, et al. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 study. J Clin Oncol. 2015;33(32):3788–95.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
Shaughnessy JN, Meena RA, Dunlap NE, et al. Efficacy of concurrent chemoradiotherapy for patients with locally recurrent or advanced inoperable breast cancer. Clin Breast Cancer. 2015;15(2):135–42.
Liu YL, Saraf A, Lee SM, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2016;157(3):555–64.
Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):128–35.
Toledano A, Garaud P, Serin D, et al. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys. 2006;65(2):324–32.
Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–91.
Printz C. Minority women more likely to develop aggressive breast cancer. Cancer. 2016;122(7):986–7.
Funding
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author EC has received funding from Merck and has served as a consultant for Eisai. Author KK has served as a consultant for Lilly, Biotheranostics, Amgen, Eisai, and Novartis. All other authors report no declarations of interest.
Ethical approval
All research was conducted in accordance with CUMC IRB approved protocol (IRB #AAAJ8512).
Informed consent
Informed consent was provided to all participants per protocol.
Rights and permissions
About this article
Cite this article
Liu, Y.L., Chin, C., Catanese, B. et al. Concurrent use of capecitabine with radiation therapy and survival in breast cancer (BC) after neoadjuvant chemotherapy. Clin Transl Oncol 20, 1280–1288 (2018). https://doi.org/10.1007/s12094-018-1859-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1859-7