Abstract
Aims
The primary standard treatment for classic Hodgkin's lymphoma (cHL) is chemotherapy and radiation therapy. However, some patients get relapsed, or their diseases become resistant. PD1 blocking antibodies have been used to increase the response of treatment in solid tumors, and led to potentially stable responses that are acceptable. Our purpose in this study is to investigate the effect of nivolumab as a PD1 blocking antibody on the survival rate of patients with Hodgkin's cancer.
Methods
Databases were found in International Medical Sciences, Web of Science, Medline, Scopus, Index Copernicus, PubMed, DOAJ, Google Scholar, EBSCO-CINAHL, and Persian databases containing SID and Magiran using keywords such as: “checkpoint inhibitor”, “nivolumab”, “Hodgkin lymphoma”, and “PD1 Blockade”. The risk of bias was determined by two external observers using the Cochrane checklists. After the search, the data provided in 51 documents was independently evaluated. Duplicate papers were excluded. Assessing the full texts of the remaining papers, 7 papers were approved.
Results
Pooled data of these seven studies revealed that the overall objective response rate was 68% (CI 64.1% to 72.1%; heterogeneity; I2 = 40.19%; p = 0.123) with partial remission (52%; CI 46.5% to 57.6%; heterogeneity; I2 = 28.36%; p = 0.212). In the pooled analysis, complete remission was 16.8 (CI 11.1% to 26.4%). Pooled data of six studies showed that stable disease was averaged to 19% (CI 16% to 23%; heterogeneity; I2 = 30%; p = 0.209; fixed-effect model).
Conclusions
The results of the study indicate that nivolumab as a PD1 pathway inhibitor can be effective in treating relapsed and refractory cHL patients compared to other therapies, and lead to more effective treatment over the long term. Furthermore, the adverse effects of nivolumab are controllable and have a good safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary standard treatment for classic Hodgkin’s lymphoma (cHL) is chemotherapy and radiation therapy. This cancer type has been reported for about 80% of patients that are being treated [1, 2]. However, some patients get relapsed, or their diseases become) resistant. Qualified patients can undergo autologous stem cell transplantation (ASCT) after chemotherapy. Clinical studies suggested that checkpoint inhibitors therapy can play a significant role in controlling malignant diseases. The goal of therapy targets immune checkpoints is to control the immune system which motivates or constrain its activities that tumors can use to protect themselves from attack by the immune system. Checkpoint therapy can prevent inhibitory checkpoints and block the restoration of the safety system. Reed–Sternberg cells use planned death (PD-1) to prevent detection by the immune system. The planned death course (PD-1) acts as an inspection point to limit immune responses by the T cells. Both PD-1 ligands, including PD-L1 and PD-L2, are interconnected to PD-1 receptors, and this interferes with PD-1 signaling, binding to T cell, activation inhibitors, and T-cell proliferation [3]. By expressing PD-1 ligands on the surface cell and PD-1 positive receptor cells, tumors can coordinate PD-1 pathway to prevent immune response [4]. PD-1 blocking antibodies have been used to increase the response of treatment of solid tumors and led to potentially stable responses that are acceptable [3,4,5,6]. The preliminary information also includes experimental degradation of PD-1 as a therapeutic strategy in some hematological cancers [7,8,9,10,11,12,13,14,15,16,17,18,19].
Genes encoding PD-1, PDL1, and PDL2 proteins are key targets for amplification of the 9p24.1 chromosome, which affects these genes in nodular sclerosis type of Hodgkin’s lymphoma. Ampillicon 9p24.1 also contains JAK2 and activates the JAK-STAT gene-linked, which results in a greater transcription of the PD-1 ligand. This copy-related mechanism results in the excessive expression of PD-1 ligands in Reed–Sternberg cells in patients with Hodgkin’s lymphoma. The Epstein–Barr virus (EBV) increases the expression of PD-1 ligands in Hodgkin’s positive EBV lymphomas. Supplementary mechanisms of excessive expression of PD-1 in Hodgkin’s lymphoma indicate that the disease can be genetically a candidate for PD-1 block [19]. Nivolumab is an IgG4 monoclonal antibody which is blocking for PD-1 and inhibits the PD-1 pathway, and it has been shown to be effective in several types of malignancy in patients who treated with nivolumab. On May 2016, after a quick priority check, the Food and Drug Administration of the United States approved the use of nivolumab for treatment of patients with classic Hodgkin’s lymphoma (cHL) who had recurrence or developmental progression after transplantation of the autologous hematopoietic stem cell transplant (HSCT). Complications which are commonly associated with PD-1 inhibitors include itching, swelling, and diarrhea. PD-1 blockers have a less toxic effect on the colitis, hepatitis, pituitaritis, and thyroiditis diseases [20,21,22].
A phase I study (CheckMate 039) conducted in the United States showed that nivolumab had an 87% effectiveness before treatment with recurrence or resistant patients with intense cHL [23]. To the best of our knowledge, a systematic review on the relationship between the response rate to treatment of patients with cHL and the nivolumab drug has not been published until today. The aim in this meta-analysis study is to investigate the effects of nivolumab on the survival rate of patients with Hodgkin’s lymphoma cancer.
Data collection
Search strategy for study selection
Databases were found in International Medical Sciences, Web of Science, Medline, Scopus, Index Copernicus, PubMed, DOAJ, Google Scholar, EBSCO-CINAHL, and Persian databases containing SID and Magiran using the following keywords: “checkpoint inhibitor”, “nivolumab”, “Hodgkin cancer”, “Hodgkin lymphoma”, “PD1 Blockade”, and “PD1”. Searches were done using Boolean operators containing “AND” and “OR” between main phrases. Furthermore, related keywords and Boolean operators were selected to change (as alternatives) the approach in each particular database.
Study selection
The search was done with two independent researchers who selected the title and the summary of all citations of their searches and selected the primary studies relevant to the systematic review topic. The literature was obtained in full text and studied independently by the two researchers. Researchers identified the primary studies and selected studies that were in line with our selection criteria. If there was a discrepancy between these two researchers, a dissenting opinion was solved using a third scholar.
Quality of research evidence
The risk of bias was determined by two external observers using the Cochrane checklists separately. Evaluation of the quality of the studies was carried out using the Cochrane Bias tool. This tool has 8 types of biases including selection bias (evaluating randomization, hiding the sequences), performance bias (blind spell checking of participants and personnel), detection bias (blind assessment of consequences of outcome), erosion bias (outcomes review), reporting bias (assessment of selected reports by author’s results), and some other biases. Base on the degree of the biases low, high, and uncertain risk, studies were considered for each part [24].
Data collection
This research was conducted by two independent researchers from December 2017 to September 2018, and the information extraction form was used for this purpose. If there was a difference between the two scholars, the difference was solved using a third scholar. The data were analyzed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist.
Data analysis
The random effects model (Der-simonian and Laird method) was applied for pooling proportions recorded across the studies. For heterogeneity evaluation, Cochrane Q test (p < 0.05 as statistically significant) and I2 indices were used.
Protocol registration
The PROSPERO code for this study was CRD42018105712.
Inclusion and exclusion criteria
Inclusion and exclusion criteria of this study are demonstrated in Table 1.
Findings
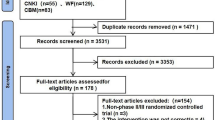
After the search, the data which was provided in 51 documents were evaluated independently. Duplicate papers were excluded, and 39 articles were evaluated. In the next phase, 32 articles which had irrelevant, case report and without the exact quantity information were excluded. Assessing the full-texts of the remaining papers, 7 papers were approved. Figure 1 displays the assessment process. Then, the key results of the designated documents were summarized (Table 2). The selected articles included a study on 17–243 participants, and a total of 560 individuals were included in the 7 eligible studies. Most of the patients in the studies received 3 mg/kg nivolumab every 2 weeks until they had complete response, cancer development, or extreme toxic effects of the drug. The average time of drug response was between 2.1 and 8.7 months. In most of the selected articles, the main outcome was the overall response rate (ORR) of nivolumab. ORR in the review studies was ranging between 64 and 95% and in all of them was above 50%. One of the evaluated outcomes in the studies was the effect of nivolumab on the progression-free survival (PFS). In these studies, it was shown that nivolumab has useful properties on PFS (between 58.25 and 86%). Adverse effects following the use of nivolumab were pyrexia (N = 3), pruritus (N = 2), rash (N = 2), hypothyroidism (N = 1), fatigue (N = 2), infusion (N = 2), neutropenia (N = 1), fever (N = 1), and increased lipase concentrations (N = 1). Bias in all papers was evaluated by use of the Cochrane instrument. Among the articles, the risk of bias was mostly unknown. There was no evidence of heterogeneity between studies; hence, fixed-effects model was chosen to pool the data. The pooled data of the seven selected studies revealed that the overall objective response rate was 68% (CI 64.1% to 72.1%; heterogeneity; I2= 40.19%; p = 0.123; Fig. 2) with partial remission (52%; CI 46.5% to 57.6%; heterogeneity; I2= 28.36%; p = 0.212; Fig. 3). In pooled analysis, complete remission (CR) was 16.8 (CI 11.1 to 26.4%; Fig. 4). Using sensitivity analysis, studies were excluded one by one to detect potential sources of high heterogeneity. The sensitivity analysis has shown that the study by Herbaux et al. [30] was a potential study. Forest plot also showed that this study was(had) the largest outlier, and it was excluded from the meta-analysis [15% (CI 12.2% to 18.6% %; heterogeneity; I2= 50.45%; p = 0.073; Fig. 2)]. The pooled data of the six studies showed that stable disease was averaged to 19% (CI 16% to 23%; heterogeneity; I2= 30%; p = 0.209; fixed-effect model; Fig. 5).
Discussion
This study was the first meta-analysis study performed on patients with relapsed and refractory cHL treated with nivolumab. The objective response rate (ORR) was 68% among 7 studies. The results of the current systematic review and meta-analysis suggest that nivolumab is an effective treatment for patients with relapsed and refractory cHL. As it was documented in the WHO 2008 classification [31], the standard treatment for patients with first recurrence of cHL is high-dose chemotherapy with autologous stem cell transplantation (ASCT) [26]. However, only 55% of patients have been shown to be free from treatment failure at 3 years [26, 32]. Patients who have recurrence after ASCT have a more severe prognosis. In these patients, Brentuximab vedotin (BV) is an important treatment option [27, 33, 34]. However, median progression-free survival (PFS) for patients treated with BV is only 3.5 months [27, 35].
The results of molecular studies indicate that patients with relapsed and refractory cHL have changes in PD1 ligand level and correspondingly, their protein expression increases the number of PDL1 and PDL2 copies in biopsied tumor cells. Increased expression of PDLl and PDL2 proteins has also been observed in Reed–Sternberg cells [23]. Nivolumab is a programmed-death blocking antibody (PD1) monoclonal antibody that inhibits T cell and strengthens the immune response against the tumor. Blocking the PD1 pathway leads to a long and adequate response to treatment in adult patients (> 18 years) with relapsed and refractory cHL after brentuximab vedotin, auto-hct treatment, and more prior lines of systemic therapy [23, 25,26,27,28,29,30].
Genes that encode PD-1, PDL1, and PDL2 proteins are key targets for enhancing the 9p24.1 chromosome. Disturbances in these genes are observed in the nodular sclerosis type of Hodgkin’s lymphoma. Base on this effect, nivolumab is expected to be suitable only for nodular nodes of Hodgkin’s lymphoma [19]. However, the results of a study in Japanese patients suggest that nivolumab is effective in treating various types of histopathologic subtypes of cHL [25].
In a number of studies, adverse effects of nivolumab in patients with relapsed and refractory cHL have been evaluated [23, 25,26,27,28,29,30]. These complications are often related to the blockage of PD1 pathway and the complications of the immune system. In the study of Armand et al. [29] the most reported complications were fatigue, diarrhea, injection-related reactions, and most of the side effects of grades 3–4 were due to drug, lipase, neutropenia, and alanine aminotransferase elevations. The most common side effect has also reported to be complication of hypothyroidism/thyroiditis and rash. The mean of the onset of the complications minimum–maximum (0–62 weeks) was 12 weeks–17 weeks (0–83 weeks). In that study, the incidence of pneumonitis was reported in two patients with grade 2 and grade 3 cancer who were both treated with corticosteroid therapy [29].
Maruyama et al. [25] reported some of the more severe complications in a number of Japanese patients which included hyponatremia, pyrexia, hepatic function abnormal, interstitial lung disease, fulminant type 1 diabetes mellitus, and rush. Most of the observed complications are related to the immune system, and they can be controlled by corticosteroids [25]. However, fulminate type 1 diabetes mellitus requires long-term treatment with insulin. This complication has been reported in the study of Armand et al. [29]. However, it has been reported that these complications may be associated with inaccurate activation of T cells in some patients with renal cell carcinoma, melanoma, and lung cessation who were treated with nivolumab and pembrolizumab [25, 36,37,38,39]. Another important complication reported in that study is interstitial lung disease [25]. Accordingly, it was suggested that physicians were aware of the risk when they prescribed anti-PD-1-antibody therapy in the patients.
Finally, the results of some studies showed safety advantageous of nivolumab in patients with relapsed/refractory cHL [23, 25,26,27,28,29,30]. In the study of Armand et al. in the United States, after 18 months of follow-up, immunological outcomes persisted and most of the adverse events were reported in grade 1 and grade 2 [29].
The use of nivolumab before allo-Hct also has been evaluated. In these patients, continuous follow up should be used to evaluate complications such as acute graft versus host disease (GVHD) grade 3 and grade 4, hepatic veno-occlusive disease, steroid-requiring febrile syndrome, and other immune-mediated adverse effects. In studies conducted in this regard, the heterogeneity of the treatment regimen was used to prevent the correct acquirement of post-transplant toxicity [23, 25,26,27,28,29,30]. Therefore, the continuation of the follow-up and the increase in the number of patients who were examined may change the post-transplant toxicity [28].
According to Herbaux’s study [30], the use of nivolumab in recurrent patients after allo-HCT treatment likely results better treatment responses than other treatments such as brentuximab (with an ORR response of 95%). However, the results of that study has reported the risk of developing acute GVHD in some patients and resulted death in two patients. All of the patients who have been diagnosed with acute GVHD and whose symptoms were observed within 1 week after the first injection of nivolumab. These results indicate that blocking the PD1 pathway in patients without a history of acute GVHD does not lead to acute GVHD. Preclinical studies and the use of fast systemic corticosteroid treatments (2 mg/kg) are advisable; therefore, it is recommended to physicians to pay attention to the risk of complications and follow-up the patients with a history of acute GVHD. In Herbaux study, after 370 days follow-up, it was not reported any chronic GVHD in recurrent after allo-HCT patients receiving nivolumab [30].
By sensitivity analysis of this study, the study by Herbaux et al. [30] was identified as a potential source of high correlation. This finding may have resulted that the Herbaux study was the only study that evaluated the effect of nivolumab in patients who were relapsing or resistant after allo-HCT treatment.
Among the limitations of this study, it can be mentioned that there were a few articles which have been studied in this subject. Most of the evaluated studies are cohort studies, and a small number of them have a control group. Additionally, in some of these studies, a number of patients have died due to illness (death due to GVHD or disease progression).
Finally, the results of this study indicate that nivolumab as a PD1 pathway inhibitor can be effective in treating relapsed and refractory cHL patients compared with other therapies, and it leads to more effective treatment over a long term. The adverse effects of nivolumab are controllable and have a good safety profile.
Conclusion
The results of this systematic review and meta-analysis revealed that nivolumab increases the survival rate of patients with relapsed and refractory cHL and its various histopathologic subtypes. Nivolumab helps to respond more effectively to long-term treatment. It has also controlled and recurrent complications in patients with relapsed/refractory cHL. This study provides enough information for oncologists to control Hodgkin cancer with combination of nivolumab and other treatments. It is suggested that in the next 2 years, another meta-analysis can be performed when more related studies are published.
References
Makita S, Maruyama D, Maeshima AM, et al. Clinical features and outcomes of 139 Japanese patients with Hodgkin lymphoma. Int J Hematol. 2016;104(2):233–44.
Ogura M, Itoh K, Kinoshita T, et al. Phase II study of ABVd therapy for newly diagnosed clinical stage II–IV Hodgkin lymphoma: Japan Clinical Oncology Group study (JCOG 9305). Int J Hematol. 2010;92(5):713–24.
Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer—preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–9.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 anti-body in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–8.
Taube JM, Klein AP, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Andorsky DJ, Yamada RE, Said J, et al. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232–44.
Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199–206.
Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51.
Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77.
Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114(10):2149–58.
Yang ZZ, Novak AJ, Stenson MJ, et al. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107(9):3639–46.
Keytruda (pembrolizumab). White-house Station, NJ: Merck (package insert). http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–77.
Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed–Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104(32):13134–9.
Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–81.
Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and post transplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8.
Kaufman D, Longo L. Hodgkin’s disease. In: Lenhord ER, Osteen R, Gansler T, editors. Clinical oncology. 2nd ed. Livingstone: Churchill; 2000. p. 2620–48.
Horning S. Hodgkin lymphoma. In: Beutler E, Lichtman M, Coller R, Kipps T, Seligsohn U, editors. Williams hematolog. 6th ed. New York: McGraw-Hill; 2001. p. 1215–28.
Fung HC, Nademanee AP. Approach to Hodgkin’s lymphoma in the new millennium. Hematol Oncol. 2002;20(1):1–15.
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9.
Abdi F, Roozbeh N. The effects of Humulus lupulus L.) hops) on menopausal vasomotor symptoms: a systematic review and meta-analysis. Iran J Obstetrics Gynecol Infer. 2016;19(26):9–17.
Maruyama D, Hatake K, Kinoshita T, et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci. 2017;108(5):1007–12.
Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94.
Beköz H, Karadurmus N, Paydas S, et al. Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Oncol. 2017;28(10):2496–502.
Kasamon YL, de Claro RA, Wang Y, et al. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical hodgkin lymphoma. Oncologist. 2017;22(5):585–91.
Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow up of the multicohort single-arm phase II Check Mate 205 trial. J Clin Oncol. 2018;36(14):1428–39.
Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkinlymphoma. Blood. 2017;129(18):2471–8.
Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Geneva: World Health Organization; 2008.
Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71.
Salihoglu A, Elverdi T, Karadogan I, et al. Brentuximab vedotin for relapsed or refractory Hodgkin lymphoma: experience in Turkey. Ann Hematol. 2015;94(3):415–20.
Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9.
Cheah CY, Chihara D, Horowitz S, et al. Patients with classical Hodgkin lymphoma experiencing disease progression after treatment with brentuximab vedotin have poor outcomes. Ann Oncol. 2016;27(7):1317–23.
Martin-Liberal J, Furness AJ, Joshi K, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64(6):765–7.
Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–7.
Okamoto M, Okamoto M, Gotoh K, et al. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7(6):915–8.
Miyoshi Y, Ogawa O, Oyama Y. Nivolumab, an anti-programmed cell death-1 antibody, induces fulminant type 1 diabetes. Tohoku J Exp Med. 2016;239(2):155–8.
Acknowledgements
We would like to thank to Dr. Ramin Sadeghi from Mashhad University Department of Nuclear Medicine for his advice in the meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval (research involving human participants and/or animals)
This work has no human or animal participants.
Informed consent
There is no consent for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amraee, A., Evazi, M.R., Shakeri, M. et al. Efficacy of nivolumab as checkpoint inhibitor drug on survival rate of patients with relapsed/refractory classical Hodgkin lymphoma: a meta-analysis of prospective clinical study. Clin Transl Oncol 21, 1093–1103 (2019). https://doi.org/10.1007/s12094-018-02032-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-02032-4