Abstract
Introduction
Hematologic toxicity (HT) in cervical cancer patients can cause treatment delays and reduction in chemotherapy, especially in high risk patients. Dose to PET-defined regions of active bone marrow (ABM) has been shown to correlate with cytopenias. An absolute volume of ABM spared may accurately represent hematopoietic reserve and risk of HT. This analysis evaluates whether the volume of ABM spared can more accurately predict HT compared to conventional dosimetric parameters.
Methods
Thirty-one patients treated for cervical cancer with chemoradiation from 9/2011 to 8/2016 were retrospectively reviewed. Receiver operating characteristic (ROC) curve were used to assess optimal cutpoint criterions for grade 3+ HT based on the CTCAEv4. Conventional dosimetric parameters to PBM and ABM (mean dose, V10, V20, V40) were assessed as well as the absolute volume (cc) of PBM and ABM spared 10, 20, and 40 Gy.
Results
The absolute volume of PBM spared 10 Gy (< 230 cc; AUC 0.732, p = 0.03) as well as volume of ABM spared 10 Gy (< 179 cc; AUC 0.815, p = 0.0002), spared 20 Gy (< 186 cc; AUC 0.774, p = 0.0015), and spared 40 Gy (< 738 cc; AUC 0.887, p < 0.0001) all predicted grade 3+ HT. In patients with < 738 cc of ABM spared 40 Gy, 18/18 (100%) had grade 3+ toxicity compared to 6/13 (46%) of patients with > 738 cc of ABM spared 40 Gy (p < 0.0001).
Conclusion
The baseline volume of ABM and the fraction of ABM present in patients vary significantly. The ongoing NRG-GY006 trial and other efforts at bone marrow sparing use V10, V20, and mean dose to the ABM during planning optimization. This analysis suggests that the volume of ABM spared 40 Gy (> 738 cc) may be a stronger predictor of HT than conventional dosimetric parameters. This should be further evaluated for clinical use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
With the onset of cytology-based Papanicolaou testing, cervical cancer incidence and mortality has steadily decreased [1]. Despite advancement in prevention and treatment, cervical cancer is the third most common cause of death among gynecologic cancers in the United States [2]. Current standard of care treatment for locally advanced cervical cancer involves definitive concurrent cisplatin chemotherapy with pelvic radiation followed by brachytherapy [3, 4]. Despite the success of this therapy, it is not without side effects. Hematologic toxicity (HT) can be a significant complication resulting in treatment delays, chemotherapy dose reductions, hospital admission, and infection risk [5,6,7]. The cause of the HT is likely multifactorial including myelosuppressive chemotherapy and pelvic bone marrow irradiation. In recent years, there have been efforts to try and reduce this toxicity while maintaining therapeutic efficacy.
The pelvis contains a substantial portion of the body’s total haematopoietically active bone marrow [6]. Bone marrow, similar to the liver or kidneys, is a parallel organ in that it is composed of many functional subunits working side by side. As long as there is sufficient volume of functional cells, toxicity will not manifest. The optimal method for defining the active marrow reserves within the bone is unknown and has been investigated for several metrics, including using bone as a surrogate volume and bifurcating the active bone marrow from the total pelvic bone volume using 18F-FDG PET imaging [6, 8]. An emerging standard in published data is using PET imaging to selectively segregate the active bone marrow fraction from the total bone using mean standard uptake values (SUV) normalized to whole body uptake, which has been shown to correlate with hematologic nadir [7]. Up to 50% of a patient’s total active bone marrow is within the pelvis and lumbar spine [7]. During pelvic irradiation for cervical cancer, a large portion of this active bone marrow is within the treatment field, which can increase the risk of HT.
Currently, there is a growing interest in the use of active pelvic bone marrow (APBM), but this is still under investigation. The optimal method for defining the active marrow reserves within the pelvic bone is unknown. Multiple surrogates have been investigated including the use of total pelvic bone and the PET-defined APBM [6, 8]. Bone marrow, much like the liver, functions as a parallel organ with toxicity resulting when a large volume of functional subunits receive a threshold dose of radiation. Therefore, an absolute volume of APBM spared may more accurately represent hematopoietic reserve and risk of HT. This analysis evaluates whether the volume of APBM spared can more accurately predict HT compared to conventional dosimetric parameters.
Methods
Thirty-one patients who received adjuvant or definitive chemoradiotherapy for a diagnosis of cervical cancer at a single institution between 2011 and 2016 were retrospectively reviewed through an IRB approved protocol. Patients were consecutively treated. All patients received concurrent weekly cisplatin (40 mg/m2). Thirteen (42%) patients received intensity-modulated radiotherapy (IMRT) while 18 (58%) received three-dimensional conformal radiotherapy. All patients received 18F-FDG PET imaging prior to chemoradiation and did not receive bone marrow stimulation during their course of treatment.
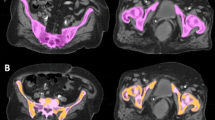
The pelvic bone marrow was defined as the contoured volumes of L4, L5, proximal femur to lesser trochanter, ilium, sacrum, ischium, and pubis using total bone as a surrogate for bone marrow, as seen in Fig. 1a. The APBM was created by first calculating the whole body mean standard uptake value (SUVwb) using the initial pretreatment PET/CT. This was done by generating a whole body contour then creating a constraint to only include the SUVs that were greater than the mean SUVwb, producing a volume defined as the constrained uptake (CU), as can be seen in Fig. 1b. The PET/CT was fused to the planning CT using MIMvista (Mimvista, Cleveland, OH) deformable registration algorithm, which was accomplished in a systematic workflow designed for this study. RegRefine was used to lock in on stable positions (sacrum and iliac crests) on the pelvis to minimize inter-patient variability in the fusion. The CU volume was transferred to the planning CT and was used to selectively isolate the bone marrow sub-volume from the PBM. The APBM volume was defined as the union of CU volume with the PBM volume (Fig. 2). The PBM and APBM volumes were collected, and the APBM/PBM fraction was calculated.
For each patient, absolute volume (cc), mean dose, V10, V20, V40, volume spared 10 Gy, volume spared 20 Gy, volume spared 40 Gy for pelvic bone marrow and active pelvic bone marrow, respectively. Complete blood count, specifically, hemoglobin (Hb), white blood cell (WBC), absolute neutrophil (ANC), and platelet count (Plt) were obtained weekly during and one week post treatment. HT was defined using the common terminology criteria for adverse events (CTCAE), version 4.03 [9]. Grade 3+ (G3+) toxicities were defined as any G3+ toxicity for Hb, ANC, Plt, WBC or lymphocyte count. Receiver operator characteristic (ROC) curves were performed comparing the predefined dosimetric continuous variables against the binary variable of any G3+ HT. Fisher’s exact test was then used to evaluate significant variables using a two-sided p value. Statistical analysis was performed using MedCalc® version 15.11.4.
Results
Patient characteristics are described in Table 1. Median age at diagnosis was 50 years (range 25–72). At presentation, 32% of patients were stage I, 29% stage II, 32% stage III and 6% stage IV. Patients were treated with a median dose of 45 Gy (range 45–68) for a median duration of 42 days (range 10–97). The mean volumes for pelvic bone marrow and active pelvic bone marrow were 1433 cc (range 901–1920) and 1098 cc (range 387–1671), respectively. The mean doses to the pelvic bone marrow and active pelvic bone marrow were 30 Gy (range 5–42) and 31 (range 5–42), respectively.
The median baseline and nadir values of hemoglobin, absolute neutrophil count, platelet and white blood cells are presented in Table 2. The primary toxicity endpoint was any G3+ HT, which occurred in 77% of the cohort. Rates of G3+ leukopenia and neutropenia were 6 and 3%, respectively. Anemia and thrombocytopenia were similar (3 and 6%, respectively).
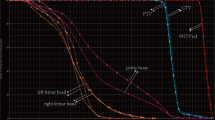
Table 3 shows the results of the ROC curve analysis. The mean dose, V10, V20, and V40 to APBM or PBM as well as the PBM spared 20 Gy and 40 Gy did not predict for G3+ HT. The absolute volume of PBM spared 10 Gy (< 230 cc; AUC 0.732, p = 0.03) as well as volume of APBM spared 10 Gy (< 179 cc; AUC 0.815, p = 0.0002), spared 20 Gy (< 186 cc; AUC 0.774, p = 0.0015), and spared 40 Gy (< 738 cc; AUC 0.887, p < 0.0001) all predicted grade 3+ HT. In patients with < 738 cc of APBM spared 40 Gy, 18/18 (100%) had grade 3+ toxicity compared to 6/13 (46%) of patients with > 738 cc of APBM spared 40 Gy (p < 0.0001, Fig. 3).
Discussion
The results of this study highlight the continued risk of hematologic toxicity in patients treated with pelvic chemoradiotherapy. This report is the first to our knowledge to evaluate the absolute volume of PET-defined active bone marrow spared in predicting hematologic toxicity in cervical cancer patients treated with chemoradiation. Compared to conventional dosimetric parameters, the volume of active bone marrow spared appears to be the strongest segregator of hematologic toxicity. One of the early studies by Klopp et al. [10] found that the V40 and mean dose to the pelvic bone marrow (PBM) correlated with higher rates of grade ≥ 2 toxicity. Further studies have shown that PBM radiation dose-volume metrics are significantly associated with weekly reductions in peripheral blood cell counts, particularly in the lower pelvis and lumbosacral spine [11].
The rates of toxicity within our cohort appear similar to those reported elsewhere within the literature. Minimizing hematologic toxicity may also allow escalated concurrent or adjuvant chemotherapy agents for high risk patients. Falcetta et al. conducted a Cochrane review, finding that adjuvant chemoradiation may improve survival with early stage cervical cancer (IA2–IIA), but has increased risk of severe HT, highlighting the importance of minimizing radiation related toxicities. Through improved dosimetric constraints, minimization of hematologic toxicity may also prevent minimize treatment breaks and chemotherapy dose reductions which can potentially compromise efficacy of therapy.
Mell et al. [12] conducted one of the earlier studies comparing the rate of HT to radiation dose to the pelvic bone marrow. The study analyzed radiation dose to the total bone marrow as well as subregions within the pelvic bone: iliac bone marrow, lower pelvis bone marrow, and lumbosacral spine bone marrow. The authors found a significant correlation between PBM V10 and grade 2+ leukopenia and neutropenia (p = 0.006). There were no associations between hemotoxicity and PMB V30 and V40. This study showed that the volume of PBM receiving low dose radiation is important in the development of HT, which supports the concept that bone marrow is a parallel organ, dependent upon a threshold of functional subunits.
Various other studies have subsequently been conducted to examine the correlation between radiation dose and volume of pelvic bone irradiated as they relate to the development of HT. These findings are summarized in Table 4. One trend in recent studies is the concept that a PET-defined, active bone marrow sub region may better predict HT. Rose et al. [7] initially reported a study evaluating the PET-defined APBM as it relates to HT. The authors found that the mean dose to the APBM significantly predicted for HT but found no association of HT in areas with PET avidity below the predefined standardized uptake value. Rose et al. concluded that the PET-defined APBM could better predict HT and help mitigate toxicity if clinically relevant constraints were determined.
There have been recent efforts to validate APBM dose constraints in patients with cervical cancer to determine if they can reduce the risk of HT. A recent INTERTECC-2 study hypothesized BM sparing IMRT could reduce acute hematologic and gastrointestinal toxicities for patients with locally advance cervical cancer [3]. The primary endpoint was the occurrence of either acute G3+ neutropenia or clinically significant GI toxicity, but there was a preplanned subgroup analysis attempting to validate PET-defined functional bone marrow sparing with IMRT. The dose constraints used for PBM and APBM were V10 < 90% and V20 < 75%. Compared with patients treated without PET-defined bone marrow sparing IMRT, those treated with this modality had significantly lower incidences of G3+ neutropenia (8.6 vs 27.1%; p = 0.035). Although useful, the optimal APBM constraint remains unknown. No studied have compared conventional dosimetric parameters to the APBM with the APBM spared.
In the current study, a comprehensive analysis of conventional dosimetric parameters to bone and APBM are compared to the absolute volume of bone and APBM spared threshold dose. The use of volume-based sparing of APBM shows promise for several reasons. First, bone is similar to liver in that it as a synthetic organ, therefore, a threshold volume spared is important in predicting toxicities. Schefter et al. [15] demonstrated this in the liver, describing low rates of hepatotoxicity with ≥ 700 cc of normal liver receiving < 15 Gy. It is rational to evaluate similar concepts in APBM sparing as this is also a synthetic organ at risk. In addition, patients in this analysis with the low volumes of active bone marrow at baseline appeared to be at the highest risk for the development of HT, which again emphasizes the importance of a volume-based model. Second, PET/CT is already commonly used with the initial staging; advances in thresholding software allow for ease of implementation. Finally, the volume of APBM may be a better predictor than PBM as a surrogate for bone marrow reserves and may be easier to avoid compared to the entire PBM while preserving target coverage and respecting other organs at risk. Volume-based metrics should be further evaluated in additional cohorts.
Our study is limited by the retrospective analysis as well as its small patient number. Despite this, a significant relationship between the volume of APBM spared threshold dose and the development of HT was observed. Although retrospective in nature, the hematologic toxicity endpoints are objective and attainable through the medical records. An inherent limitation of dosimetric analyses and hematologic toxicity assessment is that the events typically occur during the course of treatment before the total radiation dose has been delivered. Nonetheless, if these metrics can predict patients who are at the highest risk for hematologic toxicity they remain clinically useful.
Conclusion
The baseline volume of APBM and the fraction of APBM present in patients vary significantly. The ongoing NRG-GY006 trial and other studies currently use V10, V20, and mean dose to the APBM as well as PBM during planning optimization. This analysis suggests that the volume of APBM spared 40 Gy (> 738 cc) may be a stronger predictor of HT than conventional dosimetric parameters. This should be further evaluated for clinical use.
References
Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013;63(2):88–105.
Klopp AH, et al. A phase III randomized trial comparing patient-reported toxicity and quality of life (QOL) during pelvic intensity modulated radiation therapy as compared to conventional radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96(2):S3.
Mell LK, et al. Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys. 2017;97(3):536–45.
Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Preliminary analysis of RTOG 9708: adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high-risk endometrial cancer. Int J Radiat Oncol Biol Phys. 2004;59(1):168–73.
Lee J, et al. Safety and efficacy of semiextended field intensity-modulated radiation therapy and concurrent cisplatin in locally advanced cervical cancer patients: an observational study of 10-year experience. Medicine (Baltimore). 2017;96(10):e6158.
Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2003;68(3):217–26.
Rose BS, et al. Correlation between radiation dose to (1)(8)F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(4):1185–91.
Liang Y, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys. 2013;85(2):406–14.
Protocol Development | CTEP. [Online]. Available:https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 15 June 2017.
Klopp AH, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86(1):83–90.
Zhu H, et al. Longitudinal study of acute haematologic toxicity in cervical cancer patients treated with chemoradiotherapy. J Med Imaging Radiat Oncol. 2015;59(3):386–93 (quiz 394).
Falcetta FS, Medeiros LR, Edelweiss MI, Pohlmann PR, Stein AT, Rosa DD. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. 2016;11:CD005342.
Mell LK, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol. 2006;66(5):1356–65.
Albuquerque K, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow? Sparing pelvic IMRT. Int J Radiat Oncol. 2011;79(4):1043–7.
Elicin O, et al. [18F]FDG-PET standard uptake value as a metabolic predictor of bone marrow response to? Radiation: impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int J Radiat Oncol. 2014;90(2)1099–107.
Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62(5):1371–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This study was an IRB approved retrospective study.
Rights and permissions
About this article
Cite this article
Zhou, Y.M., Freese, C., Meier, T. et al. The absolute volume of PET-defined, active bone marrow spared predicts for high grade hematologic toxicity in cervical cancer patients undergoing chemoradiation. Clin Transl Oncol 20, 713–718 (2018). https://doi.org/10.1007/s12094-017-1771-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1771-6