Abstract
Objective
To delineate the association of postoperative complications with clinicopathologic factors and to identify risk factors for tumor recurrence and mortality after tumor resection in patients with colorectal cancer (CRC).
Methods
The clinical data of 1144 patients with CRC who underwent surgical intervention between 2003 and 2013 were retrieved. Correlations of postoperative complications with clinicopathologic factors were examined using univariate analysis. Risk factors for tumor recurrence and mortality of the patients after tumor resection were identified using multivariate Cox proportional hazards models. Time to relapse and overall survival were analyzed using log-rank test of Kaplan–Meier analysis.
Results
Blood carcinoembryonic antigen (CEA) significantly correlated with early symptoms, preoperative manifestations, and tumor pathology. Low differentiation grade of tumor increased the risk of recurrence after surgery in all patients with CRC. In the same cohort of patients, elevated blood CEA, low differentiation grade of tumor, laparotomy, smoking history, and TNM stage IV and III increased the mortality risk after tumor resection. In patients with advanced colon cancer, risk for postoperative mortality was increased by blood CEA, advanced tumor stage, and low tumor differentiation grade; while in those with advanced rectal cancer, blood CEA, pathologic type other than mucinous/adenocarcinoma, and laparotomy were identified as significant risk factors. In both groups of patients, postoperative chemotherapy significantly reduced the risk of mortality.
Conclusions
The present work has identified clinical factors increasing the risk of recurrence as well as mortality after surgery in more than 1,000 patients with CRC. Postoperative chemotherapy is associated with a significant reduction in the risk of mortality. All of these findings should provide insights into the better management of critically ill patients with CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) ranks third in cancer incidence globally and is the fourth leading cause of cancer-related mortality worldwide. Traditionally, CRC is highly prevalent in Western populations in North America, Australia, and West Europe; however, since more people in Asia, South America, and sub-Saharan Africa have adopted Western lifestyle and dietary habits, the number of CRC cases in these countries has gradually increased. According to the National Central Cancer Registry of China, the estimated number of new CRC cases in 2011 was 310,244, while the number of CRC-related deaths in the same year was 149,722. Several risk factors have been established for CRC including age, family history of CRC, inflammatory bowel disease, dietary habit, diabetes, obesity, and the presence of adenomatous polyps with malignant potential [1].
The molecular mechanisms of CRC initiation and progression have attracted intense investigation. It has long been accepted that colorectal tumorigenesis involves accumulation of gene mutations, chromosomal instability, and microsatellite instability [2,3,4]. In addition to genetic alternations, sustained inflammatory responses have been recently suggested to play certain roles in colon tumorigenesis [5, 6]. The complex network of genetic alternations with other pro-oncogenic pathways, however, is yet to be identified.
In developed countries, surveillance colonoscopy among high CRC risk individuals has been shown to be effective in reducing CRC incidence and mortality. The program allows removal of pre-cancerous lesions. It also ensures early detection of CRC at the asymptomatic stage, increasing the chance of successful treatments [1]. Despite the benefits of colonoscopy in cancer surveillance, many populations at high CRC risk, especially those in rural areas, do not have access to CRC screening. Indeed, only about 14% of CRC cases are diagnosed at their early stage, with a portion of patients presenting distinct metastasis, a manifestation that can significantly worsen patients’ survival [7, 8]. As such, non-invasive biomarkers and clinicopathologic factors useful for early CRC detection and stratification of CRC patients are being intensely investigated [9, 10]. Those for prompt detection of tumor recurrence after surgery are also urgently needed. In this context, in the present study, we performed a large-scale analysis of the clinical data of more than 10000 patients with CRC who underwent curative surgical resection to identify new diagnostic and prognostic markers for CRC. The analysis also uncovered clinicopathologic parameters associated with common postoperative complications, providing insights into the management of patients with CRC after surgery.
Methods
Study cohort
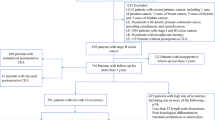
The present study reviewed the clinicopathologic data of patients with pathologically proven CRC who underwent surgical intervention between January 2003 and June 2013 at Beijing Friendship Hospital (Beijing, China). Patients from whom informed consent was not obtained were not included. A total of 1144 patients with CRC were finally enrolled. The clinicopathologic data included patients’ general background (i.e., age, gender, occupation, medical expense, and history of alcohol consumption and smoking), medical history (i.e., number of hospital admissions, history of hypertension, coronary heart disease and diabetes, personal and family history of malignancy), early symptoms (i.e., hematochezia, changes in bowel habits, and abdominal pain), preoperative clinical manifestations (i.e., preoperative distinct metastasis, preoperative hemorrhage and anemia, perforation of colon, and blood CEA), surgical parameters (i.e., type of operation and stoma), postoperative complications (i.e., pulmonary infection, bowel obstruction, hypoalbuminemia, and wound dehiscence), tumor pathology (i.e., TNM stage, differentiation, and tumor embolus), recurrence-free survival (RFS) and overall survival (OS), and chemotherapy (i.e., type and duration).
Surgical treatment
All patients underwent surgery including Dixon operation, Hartmann’s operations, Miles operation, radical laparoscopies, palliative laparoscopies, radical laparotomy, and palliative laparotomy.
Statistical analysis
Correlations between clinicopathologic variables were studied using univariate analysis, which was performed by Pearson Chi-squared or Fisher’s exact test. Risk factors for tumor recurrence and mortality were identified using Cox proportional hazards models of multivariate analysis. RFS and OS were analyzed using log-rank test of the Kaplan–Meier method. Comparison with P < 0.05 was regarded as statistically significant.
Results
Patient demographic and baseline characteristics
A total of 1144 patients with CRC were eligible for the study, including 643 males and 497 females (the gender data of 4 patients was missing). They had a mean age of 65 years. Among them, 29.3% were cigarette smokers, and 20.8% were frequent drinkers. Moreover, 36.0, 15.2, and 8.3% had hypertension, diabetes, and coronary heart disease, respectively. A small proportion of the patients (6.1%) had history of malignant diseases, and 9.1% of the cohort had a familial history of malignancy. The patients complained of hematochezia (84.5%), change in bowel habits (20.1%), and abdominal pain (23.1%). Moreover, 657 (57.4%) cases had colon cancer and 487 (42.6%) cases had rectal cancer. Most of the tumors were of stage II (38.1%) and stage III (35.0%), with over half of them (55.3%) graded as moderately differentiated (55.3%).
All patients underwent surgery. Two hundred and sixty-one (22.8%) underwent Dixon operation, 30 (2.6%) Hartmann’s operation, 100 (8.74%) Miles operation, 262 (22.9%) radical laparoscopy, 13 (1.1%) palliative laparoscopy, 355 (31.0%) radical laparotomy, and 101 (8.8%) palliative laparotomy. A total of 22 (1.9%) patients underwent other surgical approaches.
OS and RFS
After surgery, the patients were followed up, and at the time of data retrieval the surviving patients accounted for 64.6% of the cohort. Recurrence of tumor was seen in 26.8% of the patients.
Blood CEA was associated with early symptoms, preoperative clinical manifestations, and tumor pathology
CEA, which was originally extracted from human colon cancer tissues, is a tumor marker for monitoring cancer treatment and tumor recurrence after surgical resection. In addition to malignancy, CEA level is known to be elevated by other benign diseases [11]. Here, we investigated the association of blood CEA with preoperative clinical manifestations and tumor pathology using univariate analysis (Table 1). A blood CEA level of 4.6 µg/mL was used as the cutoff to stratify patients with normal and elevated CEA. High blood CEA level was associated with age (P = 0.001) and preoperative clinical manifestations including abdominal pain (P = 0.001), bowel obstruction (P = 0.001), and anemia (P < 0.0001). Of clinical importance, high blood CEA also correlated with tumor diameter (P < 0.0001), the presence of tumor nodules (P < 0.0001), distinct metastasis (P < 0.0001), status of metastatic foci (P < 0.0001), and TNM stage (P < 0.0001). High blood CEA was also associated with postoperative hypoalbuminemia (P = 0.034).
Clinicopathologic features associated with postoperative abdominal and pulmonary infection
Abdominal infection accounts for a major complication for CRC patients after surgical intervention, despite improvement in surgical procedures and patient care. Preoperative bowel obstruction was significantly correlated with postoperative abdominal infection (P < 0.0001) (Table 2). Surgical procedure likely showed potential to affect the occurrence of abdominal infection because the analysis indicated significant association between infection and stoma (P = 0.0391), type of operation (P = 0.0375), and operation method (P = 0.0013). Preoperative chemotherapy (P = 0.0401), presence of nodule (P = 0.0171), type of tumor (P = 0.0006), and stage (P = 0.0395) and grade of tumor (P = 0.0007) were associated with abdominal infection.
The potential factors linked with postoperative pulmonary infection in patients with CRC were also examined (Table 3). Patients’ age correlated with the occurrence of pulmonary infection (P = 0.0133). Infection was also associated with preoperative manifestations like bowel obstruction (P = 0.0345) and anemia (P = 0.0035). Laparotomy resulted in a higher risk of developing pulmonary infection compared to laparoscopy (P = 0.0263).
Clinicopathologic features associated with postoperative wound dehiscence/anastomotic fistula and hypoalbuminemia
Univariate analysis identified occupation as a potential factor for wound dehiscence/anastomotic fistula after surgery among CRC patients (P = 0.0308) (Table 4). Wound dehiscence/anastomotic fistula was also found to be associated with preoperative chemotherapy (P = 0.0231) and operation (P = 0.0154). The incidence of wound dehiscence/anastomotic fistula correlated with tumor type (P = 0.0011) and stage (P = 0.0497).
Clinicopathologic parameters associated with postoperative hypoalbuminemia were also identified (Table 5). A blood albumin level <30 g/L was classified as hypoalbuminemia, while the value ≥30 g/L indicated a normal albumin level. Interestingly, how the medical expenses were paid correlated with hypoalbuminemia (P = 0.0396). Preoperative bowel obstruction (P = 0.0001), hemorrhage (P = 0.0142), and blood CEA (P = 0.0283) were associated with hypoalbuminemia. Stoma and operation were two surgical parameters identified as potential factors, of which the statistical significance was 0.0336 and 0.0169, respectively. The analysis also revealed the association of hypoalbuminemia with tumor embolus (P = 0.0030), tumor nodules (P = 0.0054), tumor type (P = 0.0013) and stage (P = 0.0341), and differentiation grade (P = 0.0111).
Risk factors associated with tumor recurrence of CRC patients after surgery
Cox regression analysis identified that low (HR 3.256; P 0.0011) and medium (HR 1.908; P 0.0003) grade of tumor differentiation were two risk factors for recurrence of CRC after surgery (Table 6). Log-rank analysis showed that several factors were associated with RFS of patients with CRC (Fig. 1). The presence of distinct metastasis and non-resectable metastatic foci were the two preoperative clinical manifestations that significantly shortened RFS (P = 0.0433 or 0.0009, respectively). CRC recurrence was also associated with tumor embolus (P = 0.0083), high tumor M stage (P = 0.0196), high tumor grade (P = 0.0108), and low differentiation (P = 0.0001). Interestingly the analysis suggested that recurrence inversely correlated with tumor stage (P = 0.0153).
Risk factor associated with OS of CRC patients after surgery
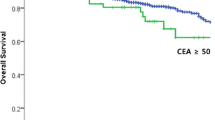
Risk factors for mortality of patients with CRC were identified using Cox regression analysis (Table 7). The factors included elevated blood CEA (HR 1.622; P 0.0004), low differentiation grade of tumor (HR 1.948; P 0.0022), laparotomy (HR 1.421; P 0.0129), smoking history (HR 1.451; P 0.0076), TNM stage IV (HR 5.018; P < 0.0001), and TNM stage III (HR 3.328; P 0.0002). Log-rank analysis was also employed to identify clinicopathologic factors associated with the OS of patients with CRC (Fig. 2). CRC patients who had suffered from malignant disease in their lifetimes before had shorter OS than those who had no history of malignancy (P < 0.0001). CRC patients whose blood CEA was elevated also had shorter OS than those with normal blood CEA level (P < 0.0001). For preoperative manifestations, obstruction (P < 0.0001), hematochezia (P = 0.001), and complaint of abdominal pain (P = 0.001) were significantly associated with poor OS after surgery. Poor prognosis after surgery was significantly associated with high tumor stage (P < 0.0001), presence of neurovascular tumor thrombi (P < 0.0001), cancer nodules (P < 0.0001), or non-resectable metastatic lesion (P < 0.0001). Palliative surgery resulted in shorter postoperative survival than radical surgery (P < 0.0001). Dismal postoperative survival was also seen in patients receiving laparotomy vs. laparoscopy (P < 0.0001). For postoperative chemotherapy, six to eight cycles of treatment achieved much better prognosis than those receiving less than two cycles of treatment (P < 0.0001). Postoperative abdominal complications or incision infection (P < 0.0001) and lung infection (P = 0.003) were associated with shorter patients’ survival after surgery.

Factors associated with postoperative survival of patients with advanced colon cancer
Clinicopathologic factors associated with the OS of patients with advanced colon cancer (stage III or IV) were studied using log-rank test of Kaplan–Meier analysis (Fig. 3). Dismal survival of the patients was associated with elevated blood level of CEA (P < 0.0001) and bowel obstruction (P = 0.002). Patients with stage IV colon cancer not surprisingly displayed worse prognosis compared to their counterparts with stage III cancer (P < 0.0001). Shorter survival was also seen in patients with low differentiation grade of tumors (P = 0.01), presence of neurovascular tumor thrombi (P = 0.017), or positive cancer nodules (P < 0.0001). The prognosis of patients with advanced colon cancer was also associated with the treatment they received. Palliative surgery of either open or laparoscopic approach resulted in shorter survival compared to radical laparotomy and radical laparoscopy (P < 0.0001). In addition, patients receiving no postoperative chemotherapy survived for a significantly shorter time than those receiving chemotherapy (P < 0.0001).
Factors associated with postoperative survival of patients with advanced rectal cancer
Clinicopathologic factors associated with the OS of patients with advanced rectal cancer (stage III or IV) were also examined using log-rank test of Kaplan–Meier analysis (Fig. 4). Similar to the situation in patients with colon cancer, poor survival after surgical intervention was associated with elevated blood CEA (P = 0.001) and bowel obstruction (P = 0.025). Dismal survival was found in patients with a history of malignancy (P = 0.043), absence of hematochezia (P = 0.002), and complaint of abdominal pain (P < 0.0001). Again, patients with stage IV cancer exhibited worse postoperative survival compared to those with stage III cancer (P = 0.022). Shorter survival was also associated with the presence of neurovascular tumor thrombi (P = 0.012) or cancer nodules (P = 0.001). Laparotomy resulted in shorter survival than laparoscopy (P < 0.0001), and similarly, palliative surgery also led to more dismal survival than the radical surgery (P < 0.0001). Patients with ostomy had shorter OS than those without ostomy (P = 0.037). Postoperative chemotherapy also significantly prolonged the OS of patients (P < 0.0001).

Risk factors for mortality after surgery in patients with advanced colon cancer
Risk factors for postoperative death of patients with advanced colon cancer were examined using Cox proportional hazards model of multivariate analysis (Table 8). Factors increasing the risk of mortality after surgery were identified as elevated blood CEA (HR 1.576; P 0.0347), stage IV cancer (HR 2.449; P 0.0004), and low tumor differentiation grade (HR 2.898; P 0.0006). Radical surgery (HR 0.478; P 0.0056) and postoperative chemotherapy (HR 0.547; P 0.0020) substantially reduced the risk of mortality.
Risk factors for mortality after surgery in patients with advanced rectal cancer
Risk factors for postoperative death of patients with advanced rectal cancer were also examined (Table 9). Factors that significantly increased the risk of mortality included elevated blood CEA (HR 1.887; P 0.0235), pathologic type other than mucinous or adenocarcinoma (HR 21.312; P 0.0223), and laparotomy vs. laparoscopy (HR 1.710; P 0.0476). Again, treatment with radical surgery (HR 0.318; P 0.0026) and postoperative chemotherapy (HR 0.445; P 0.0054) substantially reduced the risk of mortality.
Discussion
The present work retrieved and systemically analyzed the clinical data of more than 1000 patients with CRC, providing insights into the clinicopathologic features associated with early clinical symptoms, tumor pathology, and postoperative complications of patients with CRC. The study also identified risk factors for tumor recurrence and mortality of patients after surgical treatment.
CEA is a biomarker that has been employed to follow CRC for decades, but its applicability has remained controversial. A meta-analysis on published data suggested that CEA alone might not be reliable enough to detect treatable recurrences of CRC at an early stage [12]. On the other hand, CEA, when combined with other blood markers, was shown to be useful to assess the effectiveness of chemotherapy of male patients with CRC [13]. Besides, CEA is one of the factors of a prognostic score for the survival of patients with unresectable advanced CRC [14]. In this study, CEA was identified by Cox analysis as a risk factor of CRC-related death. Log-rank analysis also clearly showed that patients with CRC with elevated CEA had shorter OS compared to those with normal CEA level. These results echoed a recent study suggesting that CEA is an independent risk factor significantly affecting mortality of patients with CRC who underwent radical tumor resection [15]. The present work also revealed the potential link between CEA elevation and hypoalbuminemia after surgery. The association of CEA elevation with tumor parameters like tumor diameter identified by the present work warrants further investigation.
Despite recent advances in surgical procedures, a considerable portion of patients with CRC is inflicted with complications after surgery. A previous study suggested that for patients who underwent colorectal resection, the most frequent postoperative surgical complications are surgical site infection, anastomotic leakage, intra-abdominal abscess, ileus, and bleeding, for which the risk factors include preoperative anemia, operating time, bowel injury, obesity, prior myocardial infarction, and heart failure [16]. This work identified infections of the lungs and abdomen, hypoalbuminemia, and wound dehiscence/anastomotic fistula as major complications among the studied Chinese cohort. The clinicopathologic parameters associated with the complications were also uncovered. Of clinical importance, pulmonary infection was associated with the use of chemotherapy before surgery. In addition, interestingly, how the medical expenses were paid correlated with the occurrence of hypoalbuminemia.
The beneficial effects of laparotomy on the OS of patients with CRC are yet to be established. Studies on separated cohorts have indicated no significant differences between laparotomy and open surgery in terms of postoperative OS [17,18,19]. In contrast to these findings, the present work demonstrated that laparoscopic surgery achieved better OS of CRC patients compared to laparotomy. The reasons for the discrepancy between the present work and reported literature are unclear. Nevertheless, the usefulness of laparoscopic surgery in improving OS of CRC patients warrants further investigation.
Risk factors for the postoperative mortality of patients with advanced colon or rectal cancer have also been analyzed. The results likely provide guidance in the management of patients with late-stage colon or rectal cancer. In both groups of patients, elevation in blood CEA level was identified as a common factor increasing the mortality risk after surgery, suggesting that cancer patients with high blood CEA should be closely monitored. On the other hand, patients receiving radical surgery and postoperative chemotherapy were at a lower risk of postoperative death. The advantages of a standardized, combined use of radical surgery and postoperative chemotherapy, however, remain to be examined.
After surgical intervention, approximately 30% of patients show tumor recurrence. Using Cox analysis, the present study identified tumor embolus as a risk factor for tumor recurrence. Clinicopathologic factors like tumor pathology were also found associated with rapid tumor relapse and short OS of CRC patients after surgery. These findings are in accordance with other studies, suggesting that the aggressiveness of tumors is a significant prognostic factor of tumor recurrence and OS [20, 21]. Notably, in addition to tumor pathology, the present work revealed that hematochezia increased the risk of mortality in patients with CRC after surgery.
The present work has identified clinicopathologic factors associated with postoperative complications and uncovered the risk factors for tumor recurrence and mortality. These findings should pave the way for improvement of surgical care and prevention of recurrence and mortality of CRC patients.
References
Bonnington SN, Rutter MD. Surveillance of colonic polyps: are we getting it right? World J Gastroenterol. 2016;22(6):1925–34.
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–32.
Sameer AS, Parray FQ, Dar MA, Nissar S, Banday MZ, Rasool S, et al. Cyclin D1 G870A polymorphism and risk of colorectal cancer: a case control study. Mol Med Rep. 2013;7(3):811–5.
Sameer AS. Colorectal cancer: molecular mutations and polymorphisms. Front Oncol. 2013;3:114.
Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17(3):230–40.
Housseau F, Wu S, Wick EC, Fan H, Wu X, Llosa NJ, et al. Redundant innate and adaptive sources of IL-17 production drive colon tumorigenesis. Cancer Res. doi:10.1158/0008-5472.CAN-15-0749 (2016e-pub ahead of print Feb 15).
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71.
Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17.
Sweetser S, Jones A, Smyrk TC, Sinicrope FA. Sessile serrated polyps are precursors of colon carcinomas predominantly with deficient DNA mismatch repair. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2016.01.021 (2016e-pub ahead of print Feb 16).
Sinicrope FA, Okamoto K, Kasi PM, Kawakami H. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2016.02.008 (2016e-pub ahead of print Feb 9).
Ruibal Morell A. CEA serum levels in non-neoplastic disease. Int J Biol Mark. 1992;7(3):160–6.
Sorensen CG, Karlsson WK, Pommergaard HC, Burcharth J, Rosenberg J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence—a systematic review. Int J Surg. 2016;25:134–44.
Basu A, Seth S, Chauhan AK, Bansal N, Arora K, Mahaur A. Comparative study of tumor markers in patients with colorectal carcinoma before and after chemotherapy. Ann Transl Med. 2016;4(4):71.
Ikeguchi M, Ashida K, Saito H. New prognostic indicator is useful for predicting the survival of patients with unresectable advanced colorectal cancer. Hepatogastroenterology. 2015;62(140):859–62.
Eren T, Burcu B, Tombalak E, Ozdemir T, Leblebici M, Ozemir IA, et al. Clinical significance of the glasgow prognostic score for survival after colorectal cancer surgery. J Gastrointest Surg. doi:10.1007/s11605-016-3114-2 (2016e-pub ahead of print Feb 29).
Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4(1):5.
Pecorelli N, Amodeo S, Frasson M, Vignali A, Zuliani W, Braga M. Ten-year outcomes following laparoscopic colorectal resection: results of a randomized controlled trial. Int J Colorectal Dis. doi:10.1007/s00384-016-2587-5 (2016e-pub ahead of print Apr 18).
Patankar SK, Larach SW, Ferrara A, Williamson PR, Gallagher JT, DeJesus S, et al. Prospective comparison of laparoscopic vs. open resections for colorectal adenocarcinoma over a ten-year period. Dis Colon Rectum. 2003;46(5):601–11.
Braga M, Pecorelli N, Frasson M, Vignali A, Zuliani W, Carlo VD. Long-term outcomes after laparoscopic colectomy. World J Gastrointest Oncol. 2011;3(3):43–8.
O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008;26(14):2336–41.
Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, et al. Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery. 2007;141(1):67–75.
Acknowledgements
This work was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (ZYLX201504) and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five–year Plan (IDHT20170516).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethics approval and informed consent
The study protocol was approved by the Ethics Committee of the Second Hospital of Hebei Medical University and conducted in accordance with Helsinki’s Declaration. All the patients gave their written informed consent.
Rights and permissions
About this article
Cite this article
Bai, Z., Wang, J., Wang, T. et al. Clinicopathologic parameters associated with postoperative complications and risk factors for tumor recurrence and mortality after tumor resection of patients with colorectal cancer. Clin Transl Oncol 20, 176–192 (2018). https://doi.org/10.1007/s12094-017-1708-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1708-0