Abstract
Background
Ovarian cancer is the most lethal gynecologic malignancy worldwide with surgery as the only curative treatment. Long-term overall survival (OS) of ovarian cancer is far from satisfactory, even though significant improvement has been made in post-operative chemotherapy. One of the most important death cause is the chemoresistance due to consecutive chemotherapy. Therefore, understanding the molecular mechanisms involved in ovarian cancer development and identification of novel therapeutic targets are urgently required.
Methods
Immunohistochemical (IHC) staining was used to explore the expression pattern of mitogen-activated protein kinase (MAPK)-interacting kinase 1 (MNK1) in tumor tissues from 138 epithelial ovarian cancer (EOC) patients. Clinicopathological data were subjected to Kaplan–Meier survival and Cox multivariate analyses to evaluate the prognostic value of MNK1 in EOC. Overexpression and silencing procedures were performed on OVCAR-5 cells to investigate the mechanisms of MNK1 in regulating EOC development. The anti-tumor effects of CGP57380, a specific MNK inhibitor, were examined by cell viability assay.
Results
Higher MNK1 expression showed significant relationship with advanced FIGO stage and positive lymph node metastasis of EOC. Univariate and multivariate analyses revealed that MNK1 was an independent prognostic factor for OS of EOC patients. In vitro study demonstrated that MNK1 can promote cell proliferation through regulating the phosphorylation level of eukaryotic initiation factor 4E. In addition, inhibition of MNK1 by CGP57380 significantly down-regulated the OVCAR-5 cell viability.
Conclusion
High MNK1 expression in EOC tissues indicates poor clinical outcomes, and MNK1 can act as a potential target for novel chemotherapy development towards EOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the most lethal gynecologic malignancy worldwide [1]. The most frequent histological subtype is epithelioid ovarian cancer (EOC), accounting for over 90% of all ovarian malignancies [2]. EOC is hardly diagnosed at an early stage and, therefore, called “silent killer”. Only 15% of ovarian cancer is localized to the ovary, while about 62% occurs as distant disease [3]. Accordingly, even though significant improvements in surgical techniques and neoadjuvant chemotherapy, the 5-year overall survival (OS) for patients with the advanced disease is far from satisfactory [4]. Post-operative recurrence and drug resistance are primary causes [3]. Therefore, understanding the molecular mechanisms involved in EOC development and identification of novel therapeutic targets are urgently required.
Epithelial to mesenchymal transition (EMT) and cell proliferation are known to be modulated by PI3k/Akt/mTOR and Ras/MAPK pathways. Interestingly, both the two signaling pathways converge on mitogen-activated protein kinase (MAPK)-interacting kinase 1/2 (MNK1/2)–eukaryotic initiation factor 4E (EIF4E) axis [5]. EIF4E plays key roles in the control of mRNA translation, which can be phosphorylated and activated by MNKs [6]. The significance of EIF4E phosphorylation in cancer cell proliferation and transformation has been previously reported [7,8,9]. However, the role of its upstream regulator, MNKs, seems to be dispensable for normal cell growth and development [10]. On the other hand, MNK1, the primary isoform of MNKs which can be regulated by ERK and p38, was reported to be up-regulated in glioblastoma [11], lymphoma [12], and breast cancer [9]. However, whether MNK1 play roles in EOC is still unclear.
Since MNK1 is a downstream modulator of both mTOR and MAPK pathways, we speculated that the variable expression of MNK1 may play critical roles in EOC development and chemoresistance. In the current study, we firstly evaluated the levels of MNK1 in EOC tissues, which showed positive correlation with p-EIF4E levels. Also, increased expression of MNK1 indicates poor clinical outcomes of EOC patients, according to Kaplan–Meier survival and Cox regression analyses. Moreover, silencing or inhibition of MNK1 significantly down-regulated the proliferation capacity of OVCAR-5 cells, revealing the potential values of MNK1 inhibition in chemotherapy development for EOC patients.
Methods
Patients and specimens
We enrolled 138 patients who underwent surgical resection of primary EOC between June 2002 and June 2015 in Yidu Central Hospital of Weifang. Paraffin-embedded resected tumor tissues were obtained from the Pathology Department of our Hospital. None of the patients had serous pre-operative complications or chemotherapies before surgery. The clinicopathologic data was obtained retrospectively. The median age of the cohort was 56.5 years, with an age range of 31–85 years. The International Federation of Gynecology and Obstetrics (FIGO) stage, pre-operative CA125 level, histological type, pathological grade, and existence of lymph node metastasis of all the 138 cases were retrieved. This study was approved by The Ethics Committee of Yidu Central Hospital of Weifang. Written informed consent forms were obtained from all patients or their family members who participated in this study. The detailed patient’s characteristics are summarized in Table 1.
Antibodies and reagents
Anti-p-EIF4E (Ser209) antibody was obtained from Abcam. MNK1 (C4C1), EIF4E (C46H6), Cyclin-D1 (92G2), and MMP9 (D6O3H) antibodies were purchased from Cell Signaling. Caspase-3 (C-6), c-Myc (6A10), E-cadherin (G-10), and β-actin (I-19) antibodies were obtained from Santa Cruz Biotechnology. CGP57380 (MNK1 inhibitor) was from Sigma. SiRNAs were from Shanghai Gene Pharma Co., Ltd. (Shanghai, China).
Immunohistochemistry (IHC) and IHC evaluation
We performed immunohistochemical staining for MNK1 and p-EIF4E on 5-μm sections from the specimens. Sections were firstly incubated for 60 min at 65 °C, deparaffinized and rehydrated using a graded ethanol series. Sections were then placed in citrate buffer (pH 6.4) and microwaved for 15 min to retrieve antigen. After cooled to room temperature and washed with PBS for three times, the sections were incubated in 3% H2O2 for 20 min for quenching of endogenous peroxidase, followed by blocked in goat serum in 37 °C for 45 min. Primary antibody incubation was then performed overnight at 4 °C. The second day, sections were incubated with IgG Antibody-Fab-HRP for 30 min. Finally, DAB and Hematoxylin staining were carried out.
The IHC staining results were observed under light microscope (Olympus Corp, Tokyo, Japan). MNK1 was primarily located in nucleus of EOC cells, with slightly staining in cytoplasm. The existence of p-EIF4E was mainly observed in cytoplasm of tumor cells. Semi-quantitative expression levels were evaluated by two independent pathologists based on the average intensity and percentage of positively stained tumor cells. Staining intensity was scored as follows: 0 (negative staining), 1 (lightly yellow), 2 (deep yellow), and 3 (yellow brown). The percentage of stained tumor cells was graded as 0 (no positive cells), 1 (0–25% positive cells), 2 (25–50% positive cells), 3 (50–75% positive cells), and 4 (75–100% positive cells). Ultimate IHC score was calculated by multiplying intensity score with percentage score. IHC score >4 was classified as high expression level, while ≤4 was defined as low expression level.
Cell culture and transfections
Human ovarian carcinoma OVCAR-5 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; GE Healthcare, Logan, UT, USA), and cultured at 37 °C in a 5% CO2 atmosphere.
The construct of human MNK1 plasmid, pWZL-Neo-Myr-Flag-MNK1, was a gift from William Hahn and Jean Zhao (Addgene plasmid # 20536) [13]. For the transfection, approximately 2 × 105 cells were seeded in 6-well plates and cultured in serum-free DMEM to 40% confluence by the time of transfection. Overexpression of MNK1 was conducted by Lipofectamine 3000 reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. After 4 h of incubation, the cells were cultured in DMEM with 10% FBS and subjected to other assays.
The silencing experiments were also performed using Lipofectamine 3000 reagent. The targeting siRNAs and negative control siRNA were designed as follows:
-
EIF4E siRNA: 5′-UACAUUAAUCGGUAGCAGGAA-3′ [16]
-
Negative control siRNA (nc-siRNA): 5′-AAACCUAAGUAGAGUGGACUU-3′
RNA isolation and real-time PCR
RNA was isolated using an TRIzol Reagent Kit (Invitrogen) according to the manufacturer’s instructions. Aliquots of 1 μg total RNA were then used for quantitative reverse transcription real-time PCR analysis (qRT-PCR) by TaqMan Gene Expression Assays (Life Technologies, CA, USA). GAPDH was used an internal standard.
Specific primers for RNA quantification were designed as follows:
-
MNK1 (F): 5′-GATTCCTCTGAGACTCCAAGTTAA-3′
-
MNK1 (R): 5′-ACGCTTCTTCTTCCTCCTCTT-3′
-
MMP9 (F): 5′-CCTGGGCAGATTCCAAACCT-3′,
-
MMP9 (R): 5′-CAAAGGCGTCGTCAATCACC-3′.
-
c-Myc (F): 5′-GCCACGTCTCCACACATCAG-3′,
-
c-Myc (R): 5′-TCTTGGCAGCAGGATAGTCCT T-3′.
-
Cyclin-D1 (F): 5′-CACGCGCAGACCTTCGTT-3′,
-
Cyclin-D1 (R): 5′-GCGGATTGGAAATGAACTTCA-3′.
-
GAPDH (F): 5′-GGGTGTGAACCATGAGAAGT-3′,
-
GAPDH (R): 5′-GACTGTGGTCATGAGTCCT-3′.
Western blot
Cells were harvested with glycerol lysis buffer, boiled for 10 min, fractionated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 5% BSA in Tris-buffered saline with 1% Tween (TBST) and then incubated with primary antibodies. After washed, blots were incubated with secondary antibodies (Santa Cruz Biotechnology, USA; 1:5000 dilution) for another 45 min and finally developed using chemiluminescence reagents (Santa Cruz biotechnology, USA). Blots were later scanned and semi-quantified with Image J software.
Cell proliferation assays
The Cell Counting Kit-8 (CCK-8) assay kit (Dojindo, Kumamoto, Japan) was used to determine the impact of transfection or CGP57380 on cell proliferation. Briefly, transfected cells were plated in 96-well plates at a density of 5 × 103 cells per well for designated time points. 10 μl CCK-8 solution was added to the cells for 2 h at 37 °C, and the viability of the cells was then measured at 450 nm using a microplate reader according to the manufacturer’s instructions. Each condition was performed in triplicate, and the experiments were repeated for three times.
Statistical analysis
All statistical analyses were performed with SPSS 18.0 software. A Chi square test of cross-tab was used to evaluate the correlations between the expression of MNK1 and patient’s clinicopathologic characteristics. Survival analyses were performed with Kaplan–Meier method and log-rank test. Cox proportional hazard model was applied to evaluate the independent prognostic values of different variables. Student t test was used to analyze the significance of in vitro assays (compared with control group). P < 0.05 was considered as statistically significant.
Results
Expression of MNK1 and p-EIF4E in clinical EOC tissues
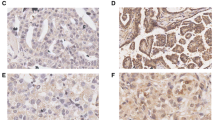
MNK1 was primarily detected in nucleus of tumor cells, while p-EIF4E was mainly located in cytoplasm (Fig. 1a–d). As described in “Methods”, high expression of MNK1 was observed in 62 cases (44.9%), and 49 cases (35.5%) showed high p-EIF4E levels. Moreover, as a result from correlation test, we found that the level of MNK1 was positively correlated with p-EIF4E level (Fig. 1e). We also collected 7 pairs of fresh-frozen tumor tissues and adjacent normal tissues to perform the RT-qPCR assay, which revealed that MNK1 was significantly higher in tumor tissues (Fig. 1f).
Expression of MNK1 and p-EIF4E in EOC tissues. a Representative IHC staining of MNK1 low expression in serous EOC. b Representative high expression of MNK1 in nucleus: the percentage of positive cells was scored as 4, and the staining intensity was scored as 3, resulting in the final IHC score with 12. d Representative low p-EIF4E level in EOC tissue. d Representative high p-EIF4E level in cytoplasm: the percentage of positive cells was scored as 4, and the staining intensity was scored as 2, resulting in the final IHC score with 8. e Cross-tab showed the significant correlation between MNK1 and p-EIF4E levels (P = 0.012). f RT-qPCR experiments revealed that the mRNA level of MNK1 was significantly higher in tumor tissues than adjacent normal tissues (P = 0.042). Scale bar 100 μm. Asterisk indicates significant difference with P < 0.05
Patients characteristics and their correlations with MNK1 expression levels
For the 138 EOC patients, 55 cases (39.9%) were diagnosed as FIGO stage I–II, and the other 83 cases (60.1%) with stage III–IV. Based on the histological type of EOC, we classified the patients into serous EOC (117 cases, 84.8%) and mucinous EOC (21 cases, 15.2%). As for pathological grades, 42 cases (30.4%) were with grade I, while 59 (42.8%) and 37 cases (26.8%) were with grades II and III, respectively. Fifty-seven patients (41.3%) occurred lymph node metastasis at the time of primary surgery.
Among those, 66 cases (47.8%) were confirmed as cancer-specific death until the end of our follow-up, and the other 72 (52.2%) were censored due to discontinued follow-up or died of reasons other than EOC or survived by the date of last follow-up. The detailed case characteristics are summarized in Table 1.
We performed Chi square test to see whether the MNK1 level has any relationship with patients clinicopathological characteristics. High MNK1 level was identified to be correlated with higher FIGO stage and positive lymph node metastasis, which revealed that MNK1 may play critical roles in EOC development and metastasis.
Univariate and multivariate analyses for prognosis of patients with EOC
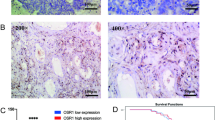
Kaplan–Meier survival analysis was carried out to estimate the prognostic values of different variables. As assumed, higher MNK1 level indicates poor OS of EOC patients (66.8 ± 4.8 vs. 90.4 ± 6.1 months, P = 0.037; Table 2; Fig. 2a). In addition, advanced FIGO stage and positive lymph node metastasis also indicated unfavorable clinical outcomes (P = 0.013 and P = 0.001, respectively; Fig. 2b, c). To better evaluate the role of MNK1 in EOC progression, we next analyzed the effect of MNK1 expression in progression-free survival (Fig. 2d), which revealed that high expression of MNK1 may also help predict disease relapse (P < 0.001).
The three prognosis factors were then subjected to Cox multivariate analysis to figuring out the independent predictive factors. Importantly, levels of MNK1 can act as an independent prognostic factor for the OS of EOC patients (HR = 1.793, 95% CI = 1.064–2.655, P = 0.038; Table 3). FIGO stage and lymph node metastasis also showed statistical significance as independent prognostic factors (P = 0.040 and P = 0.009, respectively).
MNK1 regulates the mRNA translation of proliferation-related proteins through phosphorylating EIF4E
We performed MNK1-overexpression and siRNA experiments to further investigate mechanisms of MNK1 in regulating EOC development. MNK1 silencing down-regulated the phosphorylation level of EIF4E without changing total-EIF4E level (Fig. 3a). Taking into consideration that EIF4E plays key roles in regulating protein translation, we examined the expression of proliferation-related proteins. The protein expression of Cyclin-D1, c-Myc, and MMP-9 protein all enhanced upon MNK1 overexpression; even though the mRNA levels of those molecules showed no significant changes (Fig. 3b). These results are consistent with the opinions that EIF4E mainly regulates the mRNA translation process instead of gene transcription. In addition, MNK1 also regulates E-cadherin level, indicating that MNK1 may take participate in the cell adhesion and migration. On the other hand, MNK1 showed no significant effects on the protein level of caspase-3, showing that it may not directly modulate cell apoptosis.
MNK1 regulates EOC cell proliferation through phosphorylating EIF4E. a Transfected OVCAR-5 cells were stimulated with EGF (50 ng/mL) for 30 min, and the protein levels were subsequently examined by Western blot. MNK1 can phosphorylate EIF4E without affecting total EIF4E level. MNK1 overexpression up-regulated the protein expressions of proliferation-related proteins, such as Cyclin-D1, c-Myc and MMP-9, whereas EIF4E silencing attenuated those effects. In addition, MNK1 can also negatively regulate the expression of E-cadherin, but showed no effect on Caspase-3. The effect of MNK1 inhibitor (CGP57380) was also tested paralleled. b MNK1 or EIF4E overexpression showed no direct modulation towards the mRNA levels of Cyclin-D1, c-Myc or MMP-9, according to the qRT-PCR results. c MNK1 overexpression significantly promoted OVCAR-5 cell proliferation; on the other hand, MNK1 or EIF4E silencing resulted in decreased cell viability. d Transfected cells were grown in DMEM and treated with or without 10 μmol/l CGP57380 for 5 days; cell density was then measured. CGP57380 showed comparable inhibition effects with MNK1-siRNA, indicating the potential of MNK1 as a novel chemotherapy target. In addition, the migration (e) and invasion (f) capacities of OVCAR-5 cells were both inhibited by either MNK1-siRNA or CGP57380. Asterisks indicate significant difference with P < 0.05
Moreover, EIF4E silencing can attenuate, if not abolish, the effects of MNK1 overexpression in modulating changes of those downstream proteins (Fig. 3a). Therefore, we came to the conclusion that MNK1 can mediate the expression of proliferation-related proteins through up-regulating the EIF4E phosphorylation level.
MNK1 silencing or inhibition can down-regulate EOC cell proliferation, migration and invasion
Proliferation of OVCAR-5 cells was tested upon MNK1-silencing or MNK1-overexpression. Consistent with protein expression patterns, MNK1 overexpression significantly promoted the cell proliferation process, while EIF4E-siRNA simultaneously can abolish this effect (Fig. 3c).
The in vitro results showed that MNK1 had critical functions in regulating EOC cell proliferation. Therefore, we used CGP57380, a specific MNK1 inhibitor, to further test the potential of MNK1 in chemotherapy development for EOC patients. Meaningfully, CGP57380 showed comparable effects with MNK1-silencing on inhibiting OVCAR-5 cell proliferation (Fig. 3d). We also performed scratch assay and Tranwell assay to investigate the effects of MNK1 on cell migration and invasion, respectively. Our results showed that both the migration (Fig. 3e) and invasion (Fig. 3f) capacities of OVCAR-5 cells were impaired by either MNK1-siRNA or CGP57380 treatment.
Discussion
Despite transcriptional process used to be considered as the principal control for gene expression, it is now widely approved that modulation of mRNA translation is critically involved in determining the cellular phenotype [17, 18]. Control of mRNA translation allows for rapid and spatially specific expression of proteins, which is particularly important in cancer cells [19].
EIF4E can direct ribosomes to the cap structure of mRNAs, thus functioning as the rate-limiting component of the eukaryotic translation apparatus and is involved in the mRNA-ribosome binding step of eukaryotic protein synthesis [20]. MNK1/2 was reported to phosphorylate EIF4E under the docking of EIF4G and subsequently regulate the binding activity of EIF4E [21, 22]. Moreover, MNK1-EIF4E axis was revealed as common downstream effectors of both PI3k/Akt/mTOR and Ras/MAPK signaling pathways [22,23,24], therefore, drawing considerable attentions in investigating its functions in tumor developing and treatment.
In the current study, we focused on exploring the roles of MNK1 in EOC, the most frequent cause of female cancer mortality in Western Europe and the United States [25]. Accordingly, our clinical study demonstrated that the expression level of MNK1 can serve as an independent prognostic factor for EOC; on that higher MNK1 level indicated poorer clinical outcomes. In addition, the expression level of MNK1 is positively correlated with that of p-EIF4E; however, we did not find any prognostic significance of p-EIF4E in our cohort; even it was reported that high p-EIF4E levels indicated better OS according to Noske’s study [26]. This is really interesting since that more frequent expression of total EIF4E was reported to be observed in advanced-stage EOC specimens [27]. More rigorous and larger sample analysis may be needed to illuminate the exact roles of EIF4E and its modification in EOC.
One reason for poor prognosis of EOC is due to the cancer resistance under consecutive chemotherapy, suggesting that the identification of novel rational targets for new effective therapeutic modalities is required [28]. We, therefore, tested whether MNK1 can be helpful in possible therapeutic strategies. Cell experiments demonstrated that MNK1 silencing can significantly down-regulate the expression of proliferation-related proteins, whereas up-regulate the E-cadherin level, revealing the indispensable roles of MNK1 in ovarian cancer cell proliferation and transformation. Moreover, MNK1 inhibitor, CGP57380, also showed significant inhibition for the EOC cell viability, providing new evidence for the potential of MNK1 as a chemotherapy target.
Conclusion
High MNK1 expression in EOC tissues indicates poor clinical outcomes, and inhibition of MNK1 provides novel directions for future thermotherapy development towards EOC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107.
Lalwani N, Prasad SR, Vikram R, Shanbhogue AK, Huettner PC, Fasih N. Histologic, molecular, and cytogenetic features of ovarian cancers: implications for diagnosis and treatment. Radiogr Rev Publ Radiol Soc N Am Inc. 2011;31(3):625–46. doi:10.1148/rg.313105066.
Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61(3):183–203. doi:10.3322/caac.20113.
Felix AS, Stone RA, Bowser R, Chivukula M, Edwards RP, Weissfeld JL, et al. Comparison of survival outcomes between patients with malignant mixed mullerian tumors and high-grade endometrioid, clear cell, and papillary serous endometrial cancers. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2011;21(5):877–84. doi:10.1097/IGC.0b013e31821a62dd.
Hay N. Mnk earmarks eIF4E for cancer therapy. Proc Natl Acad Sci USA. 2010;107(32):13975–6. doi:10.1073/pnas.1008908107.
Buxade M, Parra-Palau JL, Proud CG. The Mnks: MAP kinase-interacting kinases (MAP kinase signal-integrating kinases). Front Biosci J Virtual Libr. 2008;13:5359–73.
Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA. 2010;107(32):14134–9. doi:10.1073/pnas.1005320107.
Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K, Fukunaga R, et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA. 2010;107(32):13984–90. doi:10.1073/pnas.1008136107.
Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, et al. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007;21(24):3232–7. doi:10.1101/gad.1604407.
Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24(15):6539–49. doi:10.1128/mcb.24.15.6539-6549.2004.
Bell JB, Eckerdt F, Alley K, Magnusson LP, Hussain H, Bi Y, et al. MNK Inhibition Disrupts Mesenchymal Glioma Stem Cells and Prolongs Survival in a Mouse Model of Glioblastoma. Mol Cancer Res MCR. 2016. doi:10.1158/1541-7786.mcr-16-0172.
Wheater MJ, Johnson PW, Blaydes JP. The role of MNK proteins and eIF4E phosphorylation in breast cancer cell proliferation and survival. Cancer Biol Ther. 2010;10(7):728–35. doi:10.4161/cbt.10.7.12965.
Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–79. doi:10.1016/j.cell.2007.03.052.
Buxade M, Parra JL, Rousseau S, Shpiro N, Marquez R, Morrice N, et al. The Mnks are novel components in the control of TNFα biosynthesis and phosphorylate and regulate hnRNP A1. Immunity. 2005;23(2):177–89. doi:10.1016/j.immuni.2005.06.009.
O’Loghlen A, Gonzalez VM, Salinas M, Martin ME. Suppression of human Mnk1 by small interfering RNA increases the eukaryotic initiation factor 4F activity in HEK293T cells. FEBS Lett. 2004;578(1–2):31–5. doi:10.1016/j.febslet.2004.10.063.
Welnowska E, Castello A, Moral P, Carrasco L. Translation of mRNAs from vesicular stomatitis virus and Vaccinia virus is differentially blocked in cells with depletion of eIF4GI and/or eIF4GII. J Mol Biol. 2009;394(3):506–21. doi:10.1016/j.jmb.2009.09.036.
Bramham CR, Jensen KB, Proud CG. Tuning Specific Translation in Cancer Metastasis and Synaptic Memory: Control at the MNK–eIF4E Axis. Trends Biochem Sci. 2016;41(10):847–58. doi:10.1016/j.tibs.2016.07.008.
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–42. doi:10.1038/nature10098.
Jung H, Gkogkas CG, Sonenberg N, Holt CE. Remote control of gene function by local translation. Cell. 2014;157(1):26–40. doi:10.1016/j.cell.2014.03.005.
Jones RM, MacDonald ME, Branda J, Altherr MR, Louis DN, Schmidt EV. Assignment of the human gene encoding eukaryotic initiation factor 4E (EIF4E) to the region q21-25 on chromosome 4. Somat Cell Mol Genet. 1997;23(3):221–3.
Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18(1):270–9. doi:10.1093/emboj/18.1.270.
Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16(8):1909–20. doi:10.1093/emboj/16.8.1909.
Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16(12):1472–87. doi:10.1101/gad.995802.
Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428(6980):332–7. doi:10.1038/nature02369.
Wolf JK, Jenkins AD. Gene therapy for ovarian cancer (review). Int J Oncol. 2002;21(3):461–8.
Noske A, Lindenberg JL, Darb-Esfahani S, Weichert W, Buckendahl AC, Roske A, et al. Activation of mTOR in a subgroup of ovarian carcinomas: correlation with p-eIF-4E and prognosis. Oncol Rep. 2008;20(6):1409–17.
Choi CH, Lee JS, Kim SR, Lee YY, Kim CJ, Lee JW, et al. Direct inhibition of eIF4E reduced cell growth in endometrial adenocarcinoma. J Cancer Res Clin Oncol. 2011;137(3):463–9. doi:10.1007/s00432-010-0902-z.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. doi:10.1056/NEJMoa1105535.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics
Written informed consents were obtained from all the patients. The design of this study is in accordance with the guidelines of the CONSORT Statement (http://www.consort-statement.org/) for research involving human participants.
Conflict of interest
The authors have no conflicts of interest.
Additional information
S. Hou and P. Du contribute equally to this study.
Rights and permissions
About this article
Cite this article
Hou, S., Du, P., Wang, P. et al. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol 19, 1107–1116 (2017). https://doi.org/10.1007/s12094-017-1646-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1646-x