Abstract
Purpose
Changes in EGFR profiles of non small cell lung cancer (NSCLC) patients correlates to clinical outcome. Extracting quality tumor tissue remains a challenge for molecular profiling. Our study aims to ascertain the clinical relevance of urinary cell free DNA as an alternative tumor material source.
Methods
150 patients with activating EGFR mutation and received EGFR-TKIs were recruited to participate in the serial monitoring study. Matched primary tumor samples were taken together with blood and urine specimens before the initiation of TKIs. The EGFR mutation testing was performed and quantified using ddPCR. For serial time point measurements, urine and blood samples were extracted at 1-month intervals for duration of 9 months.
Results
Urinary ctDNA yielded a close agreement of 88 % on EGFR mutation status when compared to primary tissue at baseline. Almost all samples detected via urine specimens were uncovered in plasma samples. Analysis of urinary cell free DNA at different time points showed a strong correlation to treatment efficacy. Interestingly, a secondary EGFR mutation T790M was detected for 53 % of the patients during monitoring. The results were corroborated with the plasma ctDNA analysis. The T790M+ group had a reduced median survival when compared to the wildtype group.
Conclusion
Urinary cell free DNA may be a potential alternative to conventional primary tissue based EGFR mutation testing. Our findings showed that the assay sensitivity was comparable to results from blood plasma. Urinary samples being noninvasive and readily available have clinical utility for monitoring of EGFR TKI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Detection of EGFR mutations in non small cell lung cancer (NSCLC) patients is a critical step in the treatment of the disease [1]. Tyrosine kinase inhibitors (TKIs) are the standard first line treatment for patients with positive activating EGFR mutations [2]. Sensitizing EGFR mutations include the exon 19 deletions and L858R point mutation, which comprises a majority of all activating EGFR mutations [3]. However, it is not always possible to conduct EGFR mutation profiling due to the difficulties of obtaining quality biopsy samples [4, 5]. Therefore, it is important that alternative methods of acquiring tumor samples be investigated to effectively profile the disease.
A promising technique is to retrieve disease information via liquid biopsies through peripheral blood extraction [6]. For instance, circulating tumor DNA (ctDNA) present in blood plasma may shed light into the disease and can be retrieved in a less invasive method compared to conventional tissue biopsies [7]. Prior studies have determined its suitability to detect EGFR mutations in NSCLC patients [8]. Retrospective studies have shown good sensitivity for EGFR mutation detection [9] and high concordance rates when compared to primary tissues [8]. Various detection methods such as denaturing high-performance liquid chromatography [10], Scorpion-ARMS [11], BEAMING [12] and many others have shown relatively good success in measuring the quantity of ctDNA in blood specimens. The availability of substitutes for conventional methods of tumor sampling allows better-informed clinical decision making and treatment tailoring.
In our current study, we aim to further evaluate alternative samples other than primary tissue and blood plasma for possible detection and profiling of EGFR mutations. Urinary specimens have been determined to be potentially useful in a number of diseases [13] and our primary objective is to prospectively study urinary DNA in NSCLC patients to ascertain its clinical value. Urinary samples are non invasive and easily obtainable compared to blood and tissue extraction. As a result, patients more easily accept its use in disease monitoring. Our study aims to examine its diagnostics and prognostic significance by comparing with primary tissue and blood plasma in the course of EGFR-TKI treatment. Our results clearly demonstrated that the sensitivity of urinary cell free DNA is comparable to plasma cell free DNA. It also accurately tracks the dynamic changes in mutational status during treatment. Our work lays the groundwork to use urinary cell free DNA in the clinical setting for a truly noninvasive way to profile EGFR mutations in NSCLC patients.
Methods and materials
Patient cohort characteristics
All persons gave their informed consent prior to their inclusion in the study. Sample extractions were conducted via procedures approved by the institutional review board. Patients were selected randomly but adhered to the following inclusion/exclusion criteria. Patients who participated in the trial were treatment naïve and positive for the EGFR mutation L858R or L861Q. Molecular profiling was performed during routine tests on the primary tissue. Patients with de novo EGFR T790M mutations were excluded. A total of 150 patients who underwent treatment at our clinics participated in this study and the characteristics of the participants are highlighted in Table 1. Median age was 58 years old with the range between 47 and 61 year old and histology of their cancer was mostly adenocarcinoma. In addition, 50 healthy volunteers provided blood and urine specimens as negative controls for this trial. In line with guidelines, these patients were treated with 1st generation EGFR TKIs erlotinib or gefitinib. Subsequent therapies for these patients upon progression included a change to Afatinib (n = 33), continuation with their existing TKIs with/without added chemotherapy (n = 109) or other treatments (n = 8).
Study design and sample extraction
The study was conducted to determine the clinical usefulness of urinary cell free DNA in detecting EGFR mutations and also to track its active dynamic changes to EGFR-TKIs. The first sampling of peripheral blood and urine was performed prior to the start of TKI treatment. The results were directly compared to tissue samples profiles for EGFR mutations. Subsequently, sample extractions were conducted once every month during clinical checkups. Urinary samples of 30–50 ml were obtained each time, together with 3 ml of peripheral blood stored in tubes with ethylenediaminetetraacetic acid (EDTA) as anti-coagulant. Each patient was systematically profiled for duration of 9 months for L858R, L861Q and T790M.
Sample processing and ddPCR for the detection of EGFR ctDNA
Blood and urine samples were processed within 3 h of extraction to ensure the cell free DNA integrity. To extract blood plasma, centrifugation of whole blood specimens was performed for 10 min at 1000g. A repeat centrifugation step was done on the supernatant to ensure the removal of any remaining contaminating cells. For urine specimens, a single high-speed centrifugation step of 16,000g for 10 min was done and 8 ml of supernatant was recovered. Cell free DNA from both urine and plasma were purified using the Qiagen’s QIAamp Circulating Nuclei Acid kit (Qiagen Inc, US). The operating procedures followed manufacturer’s instructions and elutes were stored at −20 °C prior to the EGFR molecular test. Specimens were quantified on a Nanodrop 2000 (ThermoScientific, USA).
The EGFR mutation profiling of all samples was carried out using droplet digital PCR (ddPCR, QX200 ddPCR system, Bio-Rad Lab Inc., USA). Probes/primers for L858R, L861Q and T790M detection were obtained from Bio-Rad’s PrimePCR™ ddPCR™ Mutation Assays (Bio-Rad Lab Inc., USA). Procedures for detection followed manufacturer’s recommendations. Briefly, the reaction mix of 20 μl was setup with the cell free DNA samples from both plasma and urine specimens. Thermocycling conditions were as follows: 95 °C for 10 min followed by 40 cycles of 94 °C for 30 s and 55 °C for 1 min. Thereafter an enzyme deactivation step at 98 °C for 10 min was performed before holding the sample at 4 °C. After the PCR step, the sample plate was analyzed using the QuantaSoft software (ver 1.7, Bio-Rad Lab Inc., USA).
Statistical analysis
The correlation between urinary/plasma ctDNA with primary tissues biopsies was analyzed using the Cohen’s kappa coefficient. Comparisons of cell free DNA with healthy negative controls were performed using unpaired Student’s t test. To ascertain the clinical value of urinary cell free DNA, receiver operating curves (ROC) were constructed and the area under the curves (AUCs) were evaluated. Overall survival (OS) and progression free survival (PFS) of the patient cohort was tabulated using the Kaplan Meier survival analysis. The study also examines the hazard ratios for patients of different mutational profile using the log rank test. Unless it is otherwise specified, categorical data presented in this study are presented as mean ± standard deviation. All statistical analyses were performed using the Prism Software (GraphPad, USA).
Results
Adequacy of urinary cell free DNA
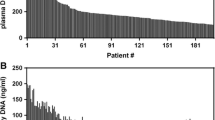
At baseline, 150 tissue samples together with matched peripheral blood and urine specimens were analyzed. The patient characteristics are highlighted in Table 1. The majority of the patients harbored L858R point mutation (89 %) and the remaining were L861Q positive. To ascertain the sufficiency of cell free DNA available for detection in urine specimens, we quantified each sample on a spectrophotometer. Figure 1 shows the comparison for urinal and plasma cell free DNA between different groups of participants. Within the patient cohort, there was no statistical difference in the quantity of cell free DNA obtained from 3 ml of peripheral blood and 8 ml of urine. The mean amount of DNA from all 150 patients was 18.5 ± 6.3 ng. In contrast, healthy volunteers registered lower quantities of both plasma and urinary cell free DNA compared to NSCLC patients. Healthy participants had a mean DNA amount of 3.7 ± 3.5 ng.
Baseline comparison of urinary circulating DNA show clinical correlates
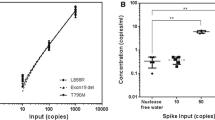
Comparative studies using urinary ctDNA with primary tissue and plasma ctDNA were performed to ascertain the sensitivity for EGFR mutation detection. Of the tissue and urinary ctDNA samples, the overall concordance rate was 88 % (κ = 0.510; 95 % CI 0.310–0.700) as shown in Fig. 2a using ddPCR. Comparing urinal and plasma ctDNA, the concordance rates were 98 % (κ = 0.900; 95 % CI 0.800–1.000) for NSCLC patients alone and 99 % when healthy volunteers were included. NSCLC patients who were L858R positive had concordance of 90 % and 98 % with matched tumor and plasma ctDNA analyses, respectively (Fig. 2b). Comparably, L861Q positive patient cohorts had 71 and 94 % concordance rates with matched tumor and plasma ctDNA, respectively. T790M mutations were not found in any of the diseased samples and control experiments involving healthy volunteers were tested negative for any EGFR mutations.
EGFR concordance measurements at baseline for 150 NSCLC patients and 50 healthy volunteers demonstrates good agreement between different sample types. a EGFR mutation status agreement between primary tissue and urinary cell free DNA. b Specific breakdown of the comparison of EGFR concordance status between different samples. c ROC analysis of urinary and plasma cell free DNA of NSCLC patients and healthy volunteers showed similar AUCs
The ROC analysis for urinal cell free DNA showed an area under the curve (AUC) of 0.976 (95 % CI 0.944–1.001) for healthy individuals and cancer patients as shown in Fig. 2c. AUC using plasma cell free DNA was 0.976 (95 % CI 0.942–1.01). Using Hanley and Mcneil method for ROC analyses comparison [14], there was no statistical significance in the AUC values using urinal and plasma cell free DNA (p value >0.05).
Tracking EGFR variations using urinal cell free DNA showed clinical response
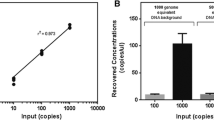
We performed serial sampling of blood and urine samples to track the dynamic changes of cell free DNA quantity and the mutational status due to EGFR-TKI treatment. Specimens were extracted at equal intervals of 1 month when possible, upon the initiation of treatment. Figure 3a, b highlights the collative results of all 150 patients in the longitudinal study for urinary and plasma cell free DNA analyses. During early phase of treatment, we observed that immediately after TKI was administered, a corresponding decline in the quantity of cell free DNA was observed irregardless of which EGFR mutation the patient had. Using a paired Student’s t test, the drop was statistically significant for both urinal (p value <0.001) and plasma (p value <0.001) cell free DNA. Of note, we observed that the decline was more pronounce in urinal cell free DNA, which registered a decrease of more than 2.8 fold.
In addition at later time points, we also detected an upward trend and a larger variation in cell free DNA quantity recovered among these patients. The fractional increase in mean plasma and urinal cell free DNA were approximately 3.7 and 4.2 folds, respectively. Further examination of the EGFR mutational profiles at the final point showed that 53 % of the cohort was positive for the secondary EGFR mutation T790M, which was absent at baseline. T790M mutation confers resistance to first generation TKI treatment [3, 15]. Therefore, we separated the cohort into two groups based on the T790M mutation status. As shown in Fig. 4, the quantity of urinary cell free DNA recovered from the T790M+ group was significantly higher as determined using a Student’s t test (p value <0.001).
Detection of T790M mutation and overall survival analysis showed urinary cell free DNA as a useful prognostic marker
The emergence of T790M mutation during TKI treatment was of concern, and henceforth, we examined each patient’s serial EGFR mutational profile in detail. We tabulated the incidence of patients who turn T790M positive at different time points (Fig. 5a) using urinary ctDNA. The first patient was detected with the secondary mutation 4 months after starting TKI treatment. The median period was 6 months for the entire T790M+ patient group. More importantly, we observed that the presence of T790M mutation was highly associated with the subsequent increase in urinary cell free DNA quantity. As clearly revealed in Fig. 5b, three representative patient profiles from the T790M+ group with the development of T790M at different time points shared the same trend of a sudden spike in cell free DNA quantity right after the detection. The trend was distinctively different from the T790M− patient group who showed little variation in DNA quantity over time.
T790M mutational status correlates to clinical outcome using urinary cell free DNA. a T790M detection frequency in the study cohort. b Individual trend lines for urinary cell free DNA measurements. Left serial measurement of three randomly selected patients in the T790M+ group. The arrows indicate the period where T790M was detected during routine urine profiling. Right serial measurements of three randomly selected patients in the T790M− group. c Overall survival of patients with differing T790M status detected using urinary cell free DNA
To verify the accuracy of the urinary ctDNA EGFR molecular genotype, we compared the results to plasma ctDNA at the final time point and matched rebiopsy tissues from a small number of consenting patients. The results are summarized in Table 2. For the plasma ctDNA analysis, the results were entirely concordant with the urinary ctDNA profiles. Comparing with matched tissue rebiopsies, we observed also good agreement with the T790M+ group. The T90M− group however had one anomaly out of the nine samples assessed (κ = 0.926). That particular sample tested positive for T790M while both the plasma and urinary ctDNA assays were negative.
Next, we investigated the prognostic role of urinary T790M status using survival analysis. OS and PFS were assessed in all 150 patients and summarized in Fig. 5c and supplementary Fig. 1. Patients in the T790M+ group had significantly worst overall survival outcome. Median OS was 30 months. The T790M− group had a median OS of 34 months. The hazard ratio determined using the log-rank test was 1.87 (95 % CI 1.08–3.24). Using the Mantel–Cox test, there is statistical significance for survival differences between the two patient groups (p value = 0.0306). For the PFS, hazard ratio was 1.16 (95 % CI 0.82–1.70). This clearly demonstrates the value for serial monitoring of the EGFR mutational status in NSCLC patients undergoing TKI treatment. Urinary cell free DNA is an attractive and noninvasive alternative to tissue analysis that will aid to track disease changes and predict survival outcome.
Discussion
Numerous studies have identified the need to accurately and rapidly track the dynamic changes in EGFR mutational profile during cancer treatment [4, 16]. Our study shows the feasibility to apply urinary cell free DNA in this endeavor. More importantly, our results are indicative that urinary cell free DNA is both prognostic and predictive of treatment response and disease outcome. This can potentially complement current disease management routines for measuring treatment efficacy and allow for treatment intervention when the patient acquires drug resistance.
The gold standard for EGFR molecular profiling of NSCLC is primary tissue biopsy and seldom repeated due to physical challenges [16]. These include patients who are too weak for repeated surgical procedures and the difficulties in getting quality specimens [5]. Our own experience concurs with these observations and patients are generally reluctant for further surgical procedures unless necessary. Liquid biopsies using plasma cell free DNA has been relatively successful as a substitute. It has demonstrated close tumor correlation [17, 18], the ability to measure drug efficacy [8] and is present in many different cancer types [19]. However, getting access to peripheral blood requires a skilled phlebotomist and the use of sharps, which can be daunting. Urinary cell free DNA is entirely noninvasive and readily available, which makes it an ideal body fluid for examination. Urine tests are common and samples are routinely used for a variety of health related detections, such as diabetes, liver disease and infection. In our study, we have demonstrated the cell free DNA that it carries is directly linked to the disease status of NSCLC patients undergoing treatment. Our results indicated a high concordance matching of the primary tissue EGFR molecular profiles. In addition, it was determined that the quality of DNA materials was comparable to plasma cell free DNA albeit at a lower concentration. AUC comparisons with plasma and urinary cell free DNA showed no significant difference in sensitivity and specificity. Processing larger volumes of urine samples as we have demonstrated can easily compensate the shortcoming.
Our study is one of the first to show the usefulness of urinary cell free DNA in treatment monitoring of NSCLC patients. Prior studies have determined its effectiveness as a marker for prostate cancer diagnosis [20], for detection of bladder cancer [21] and to detect KRAS mutation in colorectal cancer [22]. Our work further extends on existing attempts to demonstrate that urinary cell free DNA has strong associations to the disease and can be exploited to monitor the first line TKI treatment for lung cancer patients. In our analysis of all 150 patients who were treatment naïve, we observed that the mean quantity decrease more than 2.8 folds upon given TKI treatment. The cell free DNA quantity thereafter remained relatively constant for the next 4–6 months. Prior studies with clinical trials on first generation TKIs demonstrated improvement in progression free survival [3, 23]. Activating mutations such as L858R and L861Q are highlighted to play key roles in the treatment efficacy [23]. Our results are consistent with the observations of the clinical response seen by patients with these mutations, and urinary cell free DNA measurements were coherent in charting out the effects of treatment.
It is well documented that the secondary EGFR T790M mutation is a critical component that diminishes the usefulness of TKI treatments as most patients inevitable acquires the resistance mutation [24]. This will involve new classes of EGFR-irreversible inhibitors as alternative treatments and third generation TKIs have proven effective to overcome EGFR T790M [25]. The ability to detect the resistance mutation promptly is thus important and we have demonstrated active profiling captures these changes fairly early before the spike in cell free DNA content occurs. At the final time point, we observed approximately half of the cohort with the resistance EGFR T790M mutation that is consistent with published literature [24, 26]. The rate at which the mutation was picked out in the cohort varies, with the earliest positive detection at 4 months after initiation of TKI treatment. We do not observe any significant correlation of T790M positivity with gender, smoking status or age. However, the emergence of T790M led to a significant rise in the release of cell free DNA with patients registering a mean increase of 4.2 folds. The patient that registered the largest change was 13 times its lowest value right after TKI initiation. In an in vitro study, Chmielecki et al. examined the effects of the proportion of T790M clones on TKI sensitivity and accelerated cell proliferation was observed when T790M clones were greater than 25 % [27]. Lee et al. demonstrated patients with high frequencies of T790M mutations had worst clinical outcomes to TKI treatment [28], and hence our study is useful for such events to measure clinical response. Our results with increase in cell free DNA quantity would be representative of the increase in cell proportions that may have detrimental effects on clinical outcome. This elevation in cell free DNA could be linked to the increase in systemic tumor burden [29]. It will be clinically valuable to determine if such measurements provided information about the dynamics of tumor burden during treatment.
The emergence of EGFR T790M remains the hallmark for TKI resistance [3, 30] and our application using urinary ctDNA detects the mutations actively. To ascertain the link with clinical outcome, we stratified the patients based on their T790M status at the monitoring endpoint for survival analysis. Indeed, we observed worse outcome for the T790M+ group having a median survival of 4 months lesser than the T790M− cohort. This is similar to other studies using plasma ctDNA [26, 31]. For instance, Maheswaran et al. determined that the presence of T790M in pretreatment tumor biopsy specimens correlated with reduced progression free survival [31]. Our study clearly demonstrated that urinary ctDNA performs such functions well and has sufficient sensitivity and specificity compared to plasma ctDNA. Moreover, our study showed a more significant decrease in urinary cell free DNA during early phase of monitoring as a result of treatment. This would imply that urinary cell free DNA might potentially be of higher sensitivity. In addition, the extraction of urinary cell free DNA being less invasive would also be more attractive than other substitutes. For the T790M+ groups, we had also demonstrated that urinary ctDNA performed as well as rebiopsy samples and yielded 100 % concordance (Table 2). The results are indicative of the specificity of urinary ctDNA in profiling the disease. It will also allow access to tumor material where biopsies are difficult. The serial monitoring of urinary cell free DNA will bring benefits to patients on TKI treatment for molecular profiling and clinical interventions for quicker response to the dynamic changes in the disease.
The main limitation of the current methodology as we had observed in our study was the inter-patient variability in the quantity of cell free DNA. The low purity of ctDNA within each specimen was also a limiting factor. This will significantly impact the sensitivity and specificity of the assay. To gain an accurate profile of the patient’s current state, it is recommended that a higher sampling frequency be conducted to chart out the changes promptly. Urinary samples that are easily accessible will be ideal for use in constant monitoring.
Conclusion
Using a systematic approach, we established a robust means to utilize urinary cell free DNA in the disease management of NSCLC patients undergoing first line TKI treatment. The strong correlation to clinical response and the ability for active disease monitoring makes it an attractive means to probe cancer’s dynamic changes. Being truly noninvasive and with specimens’ easy access, the methodologies can be adopted simply. Our study demonstrated the critical need for clinical monitoring of EGFR mutations and showed that urinary ctDNA was sensitive and responsive to disease changes. By tracking secondary EGFR mutations, we could detect drug resistance emergence actively which led to better prediction in survival. This supports further validations using this approach in prospective clinical interventional investigations.
References
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi:10.1126/science.1099314.
Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. doi:10.1016/S1470-2045(11)70184-X.
Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–81. doi:10.1038/nrc2088.
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Am Assoc Cancer Res. 2013;19(8):2240–7.
Chouaid C, Dujon C, Do P, Monnet I, Madroszyk A, Le Caer H, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer. 2014;86(2):170–3. doi:10.1016/j.lungcan.2014.08.016.
Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472–84. doi:10.1038/nrclinonc.2013.110.
Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61(1):112–23.
Douillard J-Y, Ostoros G, Cobo M, Ciuleanu T, Cole R, McWalter G, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol. 2014;9(9):1345–53. doi:10.1097/JTO.0000000000000263.
Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698–705.
Bai H, Mao L, Wang HS, Zhao J, Yang L, An TT, et al. Epidermal growth factor receptor mutations in plasma dna samples predict tumor response in chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol. 2009;27(16):2653–9.
Kimura H, Suminoe M, Kasahara K, Sone T, Araya T, Tamori S, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA). Br J Cancer. 2007;97(6):778–84. doi:10.1038/sj.bjc.6603949.
Richardson AL, Iglehart JD. BEAMing up personalized medicine: mutation detection in blood. Clin Cancer Res. 2012;18(12):3209–11. doi:10.1158/1078-0432.CCR-12-0871.
Fischbach FT, Dunning MB. A manual of laboratory and diagnostic tests. Philadelphia: Lippincott Williams & Wilkins; 2009.
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–43. doi:10.1148/radiology.148.3.6878708.
Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–31. doi:10.1038/onc.2009.198.
Jekunen AP. Role of rebiopsy in relapsed non-small cell lung cancer for directing oncology treatments. J Oncol. 2015;2015:11. doi:10.1155/2015/809835.
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. http://www.nature.com/nm/journal/v14/n9/suppinfo/nm.1789_S1.html.
Szpechcinski A, Chorostowska-Wynimko J, Struniawski R, Kupis W, Rudzinski P, Langfort R, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer. 2015;113(3):476–83. doi:10.1038/bjc.2015.225.
Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37(3):646–50.
Casadio V, Calistri D, Salvi S, Gunelli R, Carretta E, Amadori D, et al. Urine cell-free DNA integrity as a marker for early prostate cancer diagnosis: a pilot study. BioMed Res Int. 2013;2013:5. doi:10.1155/2013/270457.
Utting M, Werner W, Dahse R, Schubert J, Junker K. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients. Am Assoc Cancer Res. 2002;8(1):35–40.
Su Y-H, Wang M, Brenner DE, Norton PA, Block TM. Detection of mutated K-ras DNA in urine, plasma, and serum of patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci. 2008;1137(1):197–206. doi:10.1196/annals.1448.027.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi:10.1056/NEJMoa040938.
Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi:10.1371/journal.pmed.0020073.
Liao B-C, Lin C-C. Yang JC-H. Second and third-generation epidermal growth factor receptor tyrosine kinase inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2015;27(2):94–101. doi:10.1097/cco.0000000000000164.
Zheng D, Ye X, Zhang MZ, Sun Y, Wang JY, Ni J et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep. 2016;6:20913. doi:10.1038/srep20913. http://www.nature.com/articles/srep20913-supplementary-information.
Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi:10.1126/scitranslmed.3002356.
Lee Y, Lee GK, Lee Y-S, Zhang W, Hwang J-A, Nam B-H, et al. Clinical outcome according to the level of preexisting epidermal growth factor receptor T790M mutation in patients with lung cancer harboring sensitive epidermal growth factor receptor mutations. Cancer. 2014;120(14):2090–8. doi:10.1002/cncr.28711.
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–90.
Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. doi:10.1056/NEJMoa044238.
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–77. doi:10.1056/NEJMoa0800668.
Acknowledgments
This work was supported by a research grant provided by the Xiangyang Central Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
All human and animal studies have been approved by the appropriate ethics committee, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
S. Chen and J. Zhao contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Zhao, J., Cui, L. et al. Urinary circulating DNA detection for dynamic tracking of EGFR mutations for NSCLC patients treated with EGFR-TKIs. Clin Transl Oncol 19, 332–340 (2017). https://doi.org/10.1007/s12094-016-1534-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1534-9