Abstract
Purpose
To analyze clinical-dosimetric predictors of genitourinary (GU) toxicity in a cohort of prostate cancer (PC) patients treated with moderate hypofractionation and simultaneous integrated boost (SIB) using volumetric modulated arc therapy (VMAT) technique.
Materials and methods
60 patients were selected. Patients were stratified into low (43 %), intermediate (30 %) and high-risk (27 %) groups. Low-risk patients received 73.5 Gy to PTV1; intermediate-risk received 73.5 Gy to PTV1 and 60 Gy to PTV2; high-risk received 73.5 Gy to PTV1, 60 Gy to PTV2, and 54 Gy to PTV3. All patients were treated in 30 fractions. Androgen deprivation therapy (ADT) was prescribed upfront in intermediate and high-risk categories. Toxicity was scored according to Common Terminology Criteria for Adverse Events v4.0 scoring system.
Results
Median follow-up was 30 months (range 16–36 months). GU acute toxicity was recorded as followS: G0 = 16/60 (27 %), G1 = 18/60 (30 %); G2 = 26/60 (43 %). GU late toxicity was recorded as follows: G0 = 20/60 (34 %); G1 = 29/60 (48 %); G2 = 11/56 (18 %). The risk of acute G2 GU toxicity was three times higher for prostate volume ≥80 cc. In 60 % of the patients with a prostate volume ≥80 cc, the first 3 weeks are at particular risk for toxicity onset. In the late setting, no statistical significance was found between GU toxicity and prostate gland dimension.
Conclusion
Prostate volume ≥80 cc resulted a predictive factor of acute G2 GU toxicity, in moderate hypofractionation and volumetric modulated arc radiation therapy for definitive PC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last years, several reports validated the impact of advanced external beam radiation treatment (EBRT) techniques and the efficacy of hypofractionated schedules for the management of localized prostate cancer (PC) in the radical setting [1–8]. Compared to 3D-conformal radiotherapy (3DCRT), intensity-modulated radiation therapy (IMRT), including rotational radiotherapy techniques, such as volumetric modulated arc therapy (VMAT), has shown to be capable of maintaining an acceptable toxicity profile and a highly conformal dose to target volume [9–11]. Studies of moderate hypofractionation combined with simultaneous integrated boost (SIB) technique, irradiating different volumes at various dose levels in the same treatment session, have confirmed benefits in clinical outcomes, due to the improvement of the therapeutic window [1, 2]. The efficacy of hypofractionation is probably related to the radiobiologic characteristics of the PC cells that demonstrated a marked sensitivity to high dose per fraction [12–16]. In this setting, different findings are available on genitourinary (GU) toxicity rates [1–8]. For these reasons, finding predictive parameters of GU toxicity could be useful for clinicians in terms of patient-tailored hypofractionation PC.
The aim of the present analysis is to identify clinical and dosimetric predictors of GU toxicity in a cohort of PC patients treated with SIB-moderate hypofractionation using VMAT technique.
Materials and methods
From January 2012, 104 patients with PC were recruited in an internal protocol of moderate SIB-hypofractionation schedule using VMAT technique (Varian RapidArc®, Palo Alto, CA, USA) for definitive treatment. Clinical and dosimetric data were prospectively collected and retrospectively analyzed.
For the intent of the present retrospective study, 60 patients out of 104 were selected. The selection criteria of the analysis were: a histologically confirmed prostate adenocarcinoma, T1–T3a, N0-1, M0, age <85 years, no recent (12 months) trans-urethral resection of the prostate (TURP), no urinary symptoms (Grade 0) at baseline evaluation according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 scoring system and an Eastern Cooperative Oncology Group (ECOG) performance status 0–1. The remaining 44 out of 104 patients were excluded because of previously submitted to TURP in the last 12 months (15 patients), presence of urinary symptoms at baseline (25 patients), ECOG performance status >1 (4 patients).
Patients’ staging was performed by pelvic magnetic resonance imaging (MRI) and/or pelvic computed tomography (CT) scan and/or 99mTc-methylene diphosphonate-planar bone scintigraphy (99mTc-MDP-BS), following the National Comprehensive Cancer Network (NCCN) recommendations. In accordance with NCCN, three risk groups were identified: low risk (clinical stage T1–T2a Gleason score ≤6 PSA ≤ 10 ng/ml), intermediate risk (clinical stage T2b-T2c or Gleason score 7 or PSA 10–20 ng/ml) and high risk (clinical stage T3a, Gleason score 8–10, PSA > 20 ng/ml).
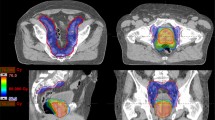
Specific recommendations were suggested regarding daily preparation: comfortably full bladder (patients were request to drink 500 ml of water just after having emptied the bladder and 30 min before each treatment session) and possibly empty rectum. Patients were submitted to planning CT in the treatment position: supine, arms on chest with Combifix™ frame (CIVCO Medical Solutions, Kalona, IA, USA). A treatment planning CT with 3 mm slice thickness, without intravenous contrast, was acquired. Delineation of the target volumes and following OARs were performed by the same radiation oncologist: rectum, bladder, intestinal cavity for bowel, femoral heads. Clinical target volume (CTV) 1 included the prostate, CTV2 consisted of CTV1 plus the entire seminal vesicles, and CTV3 consisted of CTV2 plus pelvic nodes. Planning target volumes (PTV) were defined as CTV plus 8 mm margin in all directions except posteriorly, where a 5 mm margin was used. When pelvic nodes were irradiated, an isotropic margin of 5–7 mm from pelvic lymph node CTV was used.
All patients were treated in 30 fractions as follows:
-
(a)
Low risk: a total dose of 73.5 Gy on PTV prostate only;
-
(b)
Intermediate risk: a total dose of 73.5 Gy on PTV prostate, 60 Gy on PTV seminal vesicles;
-
(c)
High risk: a total dose of 73.5 Gy on PTV prostate, 60 Gy on PTV seminal vesicles, 54 Gy on pelvis PTV.
In case of positive pelvic nodes at imaging (cN1 stage), a dose of 60 Gy was delivered to the involved nodes in SIB.
CBCTs were performed every day to verify treatment accuracy. No fiducial markers were used to assist with image guidance. Androgen deprivation therapy (ADT) was prescribed upfront in intermediate risk and 3–6 months before the radiation therapy in high-risk patients. Acute (within 90 days from the beginning of the treatment) and late side effects data were recorded using the CTCAE v4.0 scoring system.
Follow-up visits were scheduled every week during treatment and at 4 and 12 weeks after the treatment, then every 4 months for the first year and every 6 months thereafter. Dosimetric parameters were retrospectively evaluated for the statistical analysis.
Treatment planning
Treatments were delivered with 6 MV beams Trilogy™ (Varian Inc., Palo Alto, CA), equipped with a Millennium 120-MLC (multileaf collimators with 5 mm leaves in the central 20 cm). In Eclipse™ v. 10.0.28 (Varian Inc., Palo Alto, CA) treatment planning system, two-full-arcs plans were performed by RapidArc™ (Varian Inc., Palo Alto, CA) VMAT optimization (PRO™, v. 10.0.28). For all PTVs, target dose coverage for planning approval was defined by a not less than 95 % of the prescribed dose (D p) covering 95 % of the PTV volume, i.e. D 95 % ≥ 95 % D p. For PTV1 only a near maximum target dose (D 2 %) lower than 107 % D p was requested also, i.e. D 2 % ≤ 107 % D p. Finally, dose calculations were performed by AAA™ (Varian Inc., Palo Alto, CA) algorithm (v.10.0.28), with a dose calculation grid size equal to 2 mm and by including CT-based heterogeneity corrections.
The following constraints of the OARs, expressed in terms of V x (structure volume receiving at least x Gy) or D y (percentage amount y of the structure volume receiving at least the dose D Gy), had also to be satisfied for planning approval: rectum (V 46.5 < 50 %, V 64.5 < 15 %, D 1cc < 73.5 Gy), bladder (V 46.5 < 50 %, V 64.5 < 20 %, D 1cc < 73.5 Gy), penile bulb (D 1cc < 73.5 Gy), intestinal cavity (V 15 < 1050 cc, V 30 < 740 cc, V 45 < 410 cc, D 1cc < 60 Gy), and femoral heads (D 1cc < 40 Gy) [17].
Further, V x and D y values were recorded for all OARs and targets to be included in the overall analysis.
Data analysis
Concerning dosimetric parameters, bladder V x , mean dose (D mean) and maximum dose received in 1 cc (D max) values were recorded to be included in the statistical analysis.
All the variables were analyzed with Pearson’s χ 2 or Fisher’s exact tests. Prostate volumes were categorized as: ≥50 cc, ≥60 cc, ≥70 cc, ≥80 cc, ≥90 cc, and ≥100 cc. Logistic univariate analysis was performed to evaluate a correlation with acute and late GU toxicity with the following factors: prostate volume categories, bladder volume at the contouring phase (cut off values 150, 200, 250 cc), and weeks onset of toxicity.
All the volumes were based on planning CT imaging. A p value less than 0.05 was considered significant. Statistical analyses were performed using R-software 3.1.2 version.
Results
Hypofractionated treatments were completed in all patients. At a median follow-up of 30 months (range 16–36 months), one patient showed biochemical relapse during follow-up. Patient’s baseline characteristics are shown in Table 1. According to risk category, 34 (57 %) out of 60 patients were previously and/or concomitantly submitted to ADT.
Bladder dosimetric findings, stratified according to class risk groups, are summarized in Table 2. GU acute toxicity was recorded as follows: G0 = 16/60 (27 %), G1 = 18/60 (30 %); G2 = 26/60 (43 %); no case of toxicity ≥G3 was registered. GU late toxicity was recorded as follows: G0 = 20/60 (34 %); G1 = 29/60 (48 %); G2 = 11/56 (18 %); no case of toxicity ≥G3 was recorded.
The median week onset of peak acute GU toxicity was the third (range 0–4). The risk of acute G2 GU toxicity (irritative/obstructive urinary symptoms) was about three times higher in patients with a prostate volume ≥80 cc (p value 0.004; 95 % CI: 1.05–9.5). For these cases, the probability of acute G2 GU toxicity was about 60 % (p value 0.001; 95 % CI: 0.13–0.46), with an attitude to develop irritative/obstructive urinary symptoms during the first 3 weeks of treatment.
In the late setting, no statistical significance was found between GU toxicity and prostate volume.
No correlation was noted considering the prostate volume for each PC risk category. Similarly, no statistical significance was found between a bladder planning volume inferior to 150–200–250 cc with acute/late GU symptoms. Instead, considering the bladder dosimetric parameters, the only volumetric dose constraint that correlated to acute G2 GU toxicity was bladder V70 Gy ≥ 10 % (p = 0.03) with an Odds Ratio of 3.66 (95 % CI: 1.2–11.1).
No others dosimetric findings were statistically significant.
Discussion
In the present study, the potential influence of prostate volume and others clinical dosimetric predictors on GU toxicity was evaluated in a cohort of PC patients treated with a moderate SIB-hypofractionation using VMAT technique. The effect of the prostate volume on GU toxicity has been largely investigated in case of prostate brachytherapy, in which a greater risk of acute toxicity (including urinary retention and duration of retention) was reported for patients with larger prostate volume, without long-term urinary dysfunctions [18–21]. A correlation between increasing urinary toxicity and prostate volume also appears to hold true for 3DCRT and IMRT, using a conventional fractionation [22, 23]. In this setting, Pinkawa et al. assessed GU findings stratifying by prostate size [22]. In their experience, patients with a CT-scan prostate volume ≥44 cc had a significantly worse urinary subjective scores immediately after the radiation therapy, without significant effects at a median follow up of 16 months. Aizer et al. [23] retrospectively reviewed the GU toxicity of patients treated with conventional-IMRT schedule, stratified according to prostate dimension. In their experience, a CT prostate volume greater than 50 cc was related to higher acute urinary frequency/urgency and G3 GU toxicity. Moreover, patients with a prostate volume >80 cc were 9.5-fold more likely to experience acute G3 GU toxicity, according to EORTC/RTOG scale (p = 0.009).
Our findings confirmed the 80 cc as a crucial cut-off for GU irritative/obstructive symptoms within 90 days from the beginning of the treatment. In detail, statistical analysis showed that patients with a prostate volume superior or equal than 80 cc had a threefold higher risk of acute G2 GU toxicity, according to CTCAE v4.0 scale. The here reported prostate volume cut off influenced GU symptoms onset during treatment delivery. In fact, in around 60 % of cases with prostate volume superior or equal than 80 cc, the first 3 weeks are at particular risk. Similar to Pinkawa et al. [22], no significant influence on late toxicity was noted in the present population of study.
Considering the risk categories (low, intermediate and high) no difference was noted, probably related to the limited sample size. In addition, the bladder planning volume did not influence GU toxicity, probably related to the bladder inter-fraction variability, even when patients are instructed about daily preparation [24].
In downsizing prostate gland volume, the role of ADT was well recognized. Nevertheless, in specific setting, including the intermediate-risk, further investigations are demanded on its real clinical impact [25, 26]. Looking at the present findings and according to PC risk category, patients with larger prostate volume (for example ≥80 cc) could need a personalization of ADT in the neoadjuvant setting, to minimize the irritative-obstructive GU symptoms related to the prostate gland dimension.
A criticism of the current study could be the heterogeneity in adoption of MRI imaging for the treatment volumes definition. In fact, MRI was recognized in improving accuracy in prostate gland definition, reducing the target dimension by up to 30 % when compared to CT scan [27, 28]. By contrast, choline-positron emission tomography is still under investigation in the radiation treatment planning strategy [29, 30]. Unfortunately, a pretreatment MRI study could not be widely performed due to patient comorbidities, due to pace maker and/or metallic prosthesis and/or center availability. Thus, the here reported CT-based planning volumetric prostate threshold value could be useful as a predictor of moderate GU effects, even if criticisms, in the adoption of prostate MRI-study, exist.
Conclusion
Moderate hypofractionation in 30 fractions with VMAT for definitive PC was feasible and well tolerated. Acute and late toxicities were considered as mild. Larger prostate volume, greater 80 cc, resulted as predictors of moderate acute GU toxicity.
Obviously, due to the limitations of the current analysis, such as the retrospective nature and the small sample size, further investigations remain mandatory.
References
Arcangeli G, Saracino B, Gomellini S, Petrongari MG, Arcangeli S, Sentinelli S, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:11–8.
Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132–8.
Arcangeli S, Strigari L, Gomellini S, Saracino B, Petrongari MG, Pinnarò P, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–8.
Kuban DA, Nogueras-Gonzalez GM, Hamblin L. Preliminary report of a randomized dose escalation trial for prostate cancer using hypofractionation. Int J Radiat Oncol Biol Phys. 2010;78:S58–9.
Alongi F, Fogliata A, Navarria P, Tozzi A, Mancosu P, Lobefalo F, et al. Moderate hypofractionation and simultaneous integrated boost with volumetric modulated arc therapy (RapidArc) for prostate cancer. Strahlenther Onkol. 2012;188(11):990–6.
Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–8.
Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–8.
Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13:43–54.
Alongi F, Cozzi L, Fogliata A, Iftode C, Comito T, Clivio A, et al. Hypofractionation with VMAT versus 3DCRT in post-operative patients with prostate cancer. Anticancer Res. 2013;33(10):4537–43.
Di Muzio N, Fiorino C, Cozzarini C, Alongi F, Broggi S, Mangili P, et al. Phase I-II study of hypofractionated simultaneous integrated boost with tomotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:392–8.
Ferrera G, Mortellaro G, Mannino M, Caminiti G, Spera A, Figlia V, et al. Moderate hypofractionation and simultaneous integrated boost by helical tomotherapy in prostate cancer: monoinstitutional report of acute tolerability assessment with different toxicity scales. Radiol Med. 2015.
Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13.
Pedicini P, Caivano R, Strigari L, Benassi M, Fiorentino A, Fusco V, In regard to Miralbell, et al. Re: dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys. 2013;85(1):10–1.
De Bari B, Fiorentino A, Arcangeli S, Franco P, D’Angelillo RM, Alongi F. From radiobiology to technology: what is changing in radiotherapy for prostate cancer. Expert Rev Anticancer Ther. 2014;14(5):553–64.
De Bari B, Fiorentino A, Greto D, Ciammella P, Arcangeli S, Avuzzi B, et al. Prostate cancer as a paradigm of multidisciplinary approach? Highlights from the Italian young radiation oncologist meeting. Tumori. 2013;99(6):637–49.
Ritter M, Forman J, Kupelian P, Lawton C, Petereit D. Hypofractionation for prostate cancer. Cancer J. 2009;15:1–6.
Vavassori V, Fiorino C, Rancati T, Magli A, Fellin G, Baccolini M, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: results of a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2007;67(5):1401–10.
Le H, Rojas A, Hughes R, Ostler P, Lowe G, Bryant L, et al. The influence of prostate volume on outcome after high-dose-rate brachytherapy alone for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;87(2):270–4.
Monroe AT, Faricy PO, Jennings SB, Biggers RD, Gibbs GL, Peddada AV. High-dose-rate brachytherapy for large prostate volumes (> or 50 cc): uncompromised dosimetric coverage and acceptable toxicity. Brachytherapy. 2008;7:7–11.
Krupski T, Bissonette EA, Petroni GR, Theodorescu D. The impact of prostate volume following neoadjuvant androgen deprivation on quality of life and voiding symptoms in patients undergoing permanent prostate brachytherapy. Eur Urol. 2003;43:467–72.
Sherertz T, Wallner K, Wang H, Sutlief S, Russell K. Long-term urinary function after transperineal brachytherapy for patients with large prostate glands. Int J Radiat Oncol Biol Phys. 2001;51:1241–5.
Pinkawa M, Fischedick K, Asadpour B, Gagel B, Piroth MD, Nussen S, et al. Toxicity profile with a large prostate volume after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):83–9.
Aizer AA, Anderson NS, Oh SC, Yu JB, McKeon AM, Decker RH, et al. The impact of pretreatment prostate volume on severe acute genitourinary toxicity in prostate cancer patients treated with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;79(2):379–84.
Akin M, Oksuz DC, Iktueren B, Ambarcioglu P, Karacam S, Koca S, et al. Does rectum and bladder dose vary during the course of image-guided radiotherapy in the postprostatectomy setting? Tumori. 2014;100(5):529–35.
D’Angelillo RM, Franco P, De Bari B, Fiorentino A, Arcangeli S, Alongi F. Combination of androgen deprivation therapy and radiotherapy for localized prostate cancer in the contemporary era. Critical Rev Oncol Hematol. 2015;93:136–48.
Zelefsky MJ, Harrison A. Neoadjuvant androgen ablation prior to radiotherapy for prostate cancer: reducing the potential morbidity of therapy. Urology. 1997;49:38–45.
Roach M 3rd, Faillace-Akazawa P, Malfatti C, Holland J, Hricak H. Prostate volumes defined by magnetic resonance imaging andcomputerized tomographic scans for three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1996;35:1011–8.
Villeirs GM, Van Vaerenbergh K, Vakaet L, Bral S, Claus F, De Neve WJ, et al. Interobserver delineation variation using CT versus combined CT + MRI in intensity-modulated radiotherapy for prostate cancer. Strahlenther Onkol. 2005;181:424–30.
De Bari B, Alongi F, Lestrade L, Giammarile F. Choline-PET in prostate cancer management: the point of view of the radiation oncologist. Crit Rev Oncol Hematol. 2014;91:234–47.
Alongi F, Fersino S, Giaj Levra N, Mazzola R, Ricchetti F, Fiorentino A, et al. Impact of 18F-Choline PET/CT in the Decision-Making Strategy of Treatment Volumes in Definitive Prostate Cancer Volumetric Modulated Radiation Therapy. Clin Nucl Med. 2015.
Acknowledgments
The current study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Rights and permissions
About this article
Cite this article
Mazzola, R., Fersino, S., Fiorentino, A. et al. The impact of prostate gland dimension in genitourinary toxicity after definitive prostate cancer treatment with moderate hypofractionation and volumetric modulated arc radiation therapy. Clin Transl Oncol 18, 317–321 (2016). https://doi.org/10.1007/s12094-015-1371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1371-2