Abstract
Purpose
Chondrosarcoma is a malignant bone tumor with poor prognosis. Surgical treatment is the first choice for chondrosarcomas. Chondrosarcoma is not sensitive to chemotherapy and radiotherapy. Identification of biological markers is important for the early diagnosis and targeted treatment of chondrosarcoma. This study investigated the protein expression and clinicopathological significance of ROR2 and FRAT1 in 59 chondrosarcomas and 33 osteochondromas.
Methods
ROR2 and FRAT1 protein expression in tissues was measured by immunohistochemistry.
Results
The percentage of positive ROR2 and FRAT1 expression was significantly higher in patients with chondrosarcoma than in patients with osteochondroma (P < 0.01). The percentage of positive ROR2 and FRAT1 expression was significantly lower in patients with histological grade I, AJCC stage I/II stage, Enneking stage I, non-metastatic and invasive chondrosarcoma than patients with histological grade III, AJCC stage III/IV, Enneking stage II + III, metastatic and invasive chondrosarcoma (P < 0.05 or P < 0.01). ROR2 expression was positively correlated with FRAT1 expression in chondrosarcoma. Kaplan–Meier survival analysis demonstrated that histological grade, AJCC stage, Enneking stage, metastasis, invasion, and ROR2 and FRAT1 expression significantly correlated with a short mean survival time of patients with chondrosarcoma (P < 0.05 or P < 0.01). Cox multivariate analysis showed that positive ROR2 and FRAT1 expression was an independent prognostic factor that negatively correlated with postoperative survival and positively correlated with mortality.
Conclusion
Positive ROR2 and FRAT1 expression is associated with the progression and poor prognosis of chondrosarcoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondrosarcoma is a malignant bone tumor derived from cartilage cells that accounts for approximately 20 % of all malignant bone tumors [1]. In contrast, osteochondroma is the most common benign tumor of the bone derived from hyaline cartilage in the medullary cavity and periosteum [2]. Currently, surgical treatment is the preferred method for effective treatment of chondrosarcoma and osteochondroma. Chondrosarcoma is not sensitive to radiotherapy and chemotherapy. Therefore, patients with metastatic tumors or tumors that occur in specific locations of the body that prevent surgical resection often have poor prognosis [1]. Unfortunately, molecular therapy of chondrosarcoma has not been effectively established in the clinic. Therefore, identification of biomarkers that can be developed into a target for molecular therapy or a predictive factor of prognosis is still important for the treatment of chondrosarcoma [3].
Wnt signaling is essential for cellular proliferation, survival, migration, and adhesion, which are important functions necessary for tumorigenesis and metastasis [4]. Deregulation of Wnt signaling has been observed in a variety of cancers [5]. ROR2 (receptor tyrosine kinase-like orphan receptor 2) is a member of the (RTKs) receptor tyrosine kinase family and the receptor of Wnt5a. ROR2 is involved in the regulation of JAK-STAT3 and Wnt/JNK signaling pathway [4, 6]. Recent studies have found abnormal ROR2 expression in a variety of tumor cells, which is believed to be associated with its pro-oncogenic and tumor suppressive roles [4, 6]. FRAT1 (frequently rearranged in advanced T-cell lymphomas 1) is a proto-oncogene encoding the FRAT1 protein, which is a positive regulatory factor of the Wnt/β-catenin signaling pathway [7, 8]. Overexpression of FRAT1 may cause aberrant activation of Wnt signaling. Recent studies have observed high FRAT1 expression in numerous cancers and found that FRAT1 is involved in the progression and prognosis of these cancers. However, the expression of ROR2 and FRAT1 in chondrosarcoma and osteochondroma has not been reported.
In this study, the expression of ROR2 and FRAT1 proteins in chondrosarcoma and osteochondroma was investigated using immunohistochemical methods. The clinical and pathological significance of ROR2 and FRAT1 protein as well as their predictive role in the prognosis of patients with chondrosarcoma was analyzed.
Materials and methods
Specimens and clinical data
Resected tumor specimens of 59 patients with chondrosarcoma were collected from January 2001 to June 2011 at the Second Xiangya Hospital and Third Xiangya Hospital, Central South University. The diagnosis was confirmed by histopathology. The clinicopathological characteristics of 59 patients with chondrosarcoma are presented in Table 1. Survival information of 59 patients with chondrosarcoma was obtained with the longest follow-up being 134 months; 13 patients died during the follow-up period (22.0 %), while 46 patients survived (i.e. censored cases) (78.0 %). Osteochondroma specimens were collected from 33 patients including 27 males (81.8 %) and 6 females (18.2 %). Of the 33 patients, 29 patients were aged 45 years or younger (87.9 %), while 4 patients were older than 45 (12.1 %); 28 patients had a tumor mass with a maximal diameter ≤5 cm (84.8 %) and 5 patients had a tumor with a diameter >5 cm (15.2 %).
Immunohistochemistry
Rabbit anti-human ROR2 and FRAT1 polyclonal antibodies were purchased from Abgent Company (Califorina, CA, USA). EnVisionTM Detection kit was purchased from Dako Laboratories (California, CA, USA). The positive controls were the positive breast cancer sections provided by Abgent Company. EnVision immunohistochemical staining was performed according to the manufacturer’s protocol. Briefly, 4 µM-thick sections were cut from routinely paraffin-embedded tissues. The sections were deparaffinized and then incubated with peroxidase inhibitor (3 % H2O2) in the dark for 15 min. Next, the sections were incubated with rabbit anti-human ROR2 and FRAT1 primary antibody for 60 min. After being soaked with PBS for 3 × 5 min, Solution A was added to the sections for 30 min followed by DAB staining and haematoxylin counter-staining. The slides were then dehydrated with alcohol, soaked in xylene, and mounted with neutral balsam. 400 cells in 10 random fields were examined per section. Patients with positive cells ≥25 % was considered positive whereas everything else was considered negative.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences version 18.0 (SPSS 18.0). The inter-relationship of ROR2 and FRAT1 expression with histological or clinical factors was analyzed using \( \chi^{2} \) or Fisher’s exact test. Kaplan–Meier and log-rank tests were used for univariate survival analysis. Cox proportional hazards model was used for multivariate analysis and determining the 95 % confidence interval. A P < 0.05 was considered statistically significant.
Results
ROR2 and FRAT1 protein expression in chondrosarcoma and osteochondroma tissues
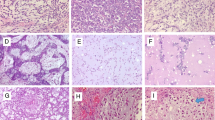
Positive ROR2 and FRAT1 staining was mainly located in the cytoplasm, and less at the nucleus of tumor cells (Figs. 1, 2). Of the 59 chondrosarcomas, positive ROR2 staining was observed in 39 tissues (66.1 %), and positive FRAT1 staining was observed in 34 tissues (57.6 %). Of the 33 osteochondromas, positive ROR2 staining was observed in 3 patients (9.1 %), and positive FRAT1 was observed in 3 patients (9.1 %). The percentage of positive ROR2 and FRAT1 expression in patients with chondrosarcoma was significantly higher than that in patients with osteochondroma (P = 0.000).
Immunohistochemical staining of ROR2 expression. Original magnification ×200. Positive reaction was mainly localized in the cytoplasm. a Positive ROR2 expression in chondrosarcoma tissues. b Negative ROR2 expression in chondrosarcoma tissues. c Positive ROR2 expression in osteochondroma tissues. d Negative ROR2 expression in osteochondroma tissues
Immunohistochemical staining of FRAT1 expression. Original magnification ×200. Positive reaction was mainly localized in the cytoplasm. a Positive FRAT1 expression in chondrosarcoma tissues. b Negative FRAT1 expression in chondrosarcoma tissues. c Positive FRAT1 expression in osteochondroma tissues. d Negative FRAT1 expression in osteochondroma tissues
Association of ROR2 and FRAT1 expression with the clinicopathological features of chondrosarcoma
As shown in Table 2, the percentage of positive ROR2 and FRAT1 expression in patients with histological grade I, AJCC stage I + II, Enneking stage I, non-metastatic and invasive chondrosarcomas was significantly lower than that in patients with histological grade III, AJCC stage III + IV, Enneking stage II + III, metastatic and invasive chondrosarcomas (P < 0.05 or P < 0.01). No significant association between ROR2 and FRAT1 expression and the age, gender, histological type, or maximum tumor diameter was observed in patients with chondrosarcoma (P > 0.05). Of the 39 chondrosarcomas with positive ROR2 expression, 29 patients were FRAT1 positive. Of the 20 patients with negative ROR2 expression, 15 were FRAT1 negative. A significantly positive correlation between the expression of ROR2 and FRAT1 was observed (P = 0.000).
Correlations between clinicopathological parameters, ROR2 and FRAT1 expression, and the mean survival of patients with chondrosarcoma were observed
Survival information of 59 cases with chondrosarcoma was collected via letter or telephone interviews. 13 cases (22.0 %) died during the follow-up of 134 months. Kaplan–Meier survival analysis showed that the gender, histological grade, AJCC stage, Enneking stage, metastasis, and invasion were significantly associated with the mean survival time of patients with chondrosarcoma (Table 3. P < 0.05 or P < 0.01). Patients with positive ROR2 and FRAT1 expression survived significantly shorter than patients with negative ROR2 and FRAT1 expression (Table 3 & Fig. 3. P < 0.05).
ROR2 and FRAT1 expression and survival in patients with chondrosarcoma. a Kaplan–Meier plots of mean survival in patients with chondrosarcoma and with ROR2 positive and negative expression. b Kaplan–Meier plots of mean survival in patients with chondrosarcoma and with FRAT1 positive and negative expression
Cox multivariate analysis showed that histological grade III, AJCC stage III or IV, Enneking stage III, tumor metastasis, invasion, and positive ROR2 or FRAT1 expression negatively correlated with postoperative survival rate and positively correlated with mortality (Table 3). Positive ROR2 and FRAT1 expression was an independent prognostic factor in patients with chondrosarcoma (Table 4).
Discussion
Current therapy of chondrosarcoma includes surgical resection and chemotherapy. However, patients with metastatic tumors or resistance to initial chemotherapy often have poor prognosis [9]. In addition, conventional chemotherapy is highly toxic. Therefore, various new therapeutic approaches have been developed in the past decade to target tumor cells by interfering with signaling pathways involved in cell proliferation, survival, or chemoresistance [4, 5]. However, effective targeting therapy against tumor cells of chondrosarcoma has not yet been well established in the clinic. In this study, we identified high expression of ROR2 and FRAT1 protein in chondrosarcoma. Both positive ROR2 and FRAT1 expression are associated with malignancy of chondrosarcoma and poor prognosis of patients with chondrosarcoma. Low expression of ROR2 and FRAT1 was observed in benign tumors of the bone, which implicates the value of ROR2 and FRAT1 as a target for targeted therapy of chondrosarcoma.
Recent studies have found high expression of ROR2 in some malignancies, such as melanoma [10], renal cell carcinoma [11], prostate carcinoma [12], and stromal tumors [13]. The high expression of ROR2 is associated with poor differentiation, high clinical stage, susceptibility to metastasis and invasion, or poor prognosis of these malignancies. Recently, two groups reported a correlation between high ROR2 expression and disease severity in osteosarcoma [14, 15]. ROR2’s oncogenic role is supposed to be associated with Wnt5a-dependent cell proliferation, migration, and invasion [4]. In contrast, the tumor suppressive role of ROR2 has been reported in colon cancer [16], hepatocellular cancer [17], and multiple hematological malignancies [18]. ROR2 expression was significantly downregulated or lost in these cancer cells. The tumor suppression of ROR2 is hypothesized to be associated with its ability to inhibit the pro-tumorigenic effects of the canonical Wnt pathway [4]. This study observed high ROR2 expression in chondrosarcoma, which was associated with the disease severity and poor prognosis, suggesting that ROR2 plays oncogenic roles in chondrosarcoma. The role of ROR2 in chondrosarcoma may be associated with its ability in regulating cell proliferation, differentiation, adhesion, migration, and invasion processes [19, 20].
Proto-oncogene FRAT1 encodes a 29kD protein [8, 21] and is first identified as T-cell lymphoma proto-oncogene, which positively regulates Wnt signal transduction pathway [21, 22]. FRAT1 inhibits the role of GSK3 on β-catenin phosphorylation by binding to GSK3 and subsequently elevating the cytoplasmic levels of free β-catenin. β-catenin enters into the nucleus, combines with members of the TCF/LEF family, and then activates the target genes [8, 21, 22]. Previous studies demonstrated that FRAT1 is overexpressed in esophageal cancer [23], lung cancer [24], ovarian cancer [25], Glioma [25], and glioblastoma [26]. The high expression of FRAT1 is associated with high malignancy, disease severity, and/or poor prognosis of these cancers. However, the expression of FRAT1 in both chondrosarcoma and osteochondroma has not been reported. In this study, we demonstrated that FRAT1 was highly expressed in chondrosarcoma, which was associated with tumor metastasis, invasion, high pathological grade and clinical stage, and short postoperative survival.
This study also found that percentage of positive ROR2 and FRAT1 expression was significantly lower in osteochondroma (9.1 %) than that in chondrosarcoma (66.1 and 57.6 %, respectively, P = 0.000). Moreover, a significantly positive correlation between the expression of ROR2 and FRAT1 was observed (P = 0.000). These findings suggest that both ROR2 and FRAT1 may be involved in the tumorigenesis of bone malignancies, and these two proteins may collaborate during the development of chondrosarcoma through regulating Wnt signaling. Therefore, ROR2 and/or FRAT1 could be used as biomarkers for the potential for aggressive behavior or metastasis.
In conclusion, positive FRAT1 and ROR2 expression is a biomarker for disease severity and a predictive factor of poor prognosis in patients with chondrosarcoma.
References
Pritchard DJ, Lunke RJ, Taylor WF, Dahlin DC, Medley BE. Chondrosarcoma: a clinicopathologic and statistical analysis. Cancer. 1980;45:149–57.
Sekharappa V, Amritanand R, Krishnan V, David KS. Symptomatic solitary osteochondroma of the subaxial cervical spine in a 52-year-old patient. Asian Spine J. 2014;8:84–8.
Kim MJ, Cho KJ, Ayala AG, Ro JY. Chondrosarcoma: with updates on molecular genetics. Sarcoma. 2011;2011:405437.
Ford CE, Qian Ma SS, Quadir A, Ward RL. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int J Cancer. 2013;133:779–87.
Niehrs C. The complex world of WNT receptor signaling. Nat Rev Mol Cell Biol. 2012;13:767–79.
Ren D, Minami Y, Nishita M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following snail-mediated epithelial-mesenchymal transition. Genes Cells. 2011;16:304–15.
Saitoh T, Mine T, Katoh M. Molecular cloning and expression of proto-oncogene FRAT1 in human cancer. Int J Oncol. 2002;20:785–9.
van Amerongen R, Nawijn MC, Lambooij JP, Proost N, Jonkers J, Berns A. Frat oncoproteins act at the crossroad of canonical and noncanonical Wnt-signaling pathways. Oncogene. 2010;29:93–104.
Heymann D, Rédini F. Targeted therapies for bone sarcomas. Bonekey Rep. 2013;2:378.
O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, et al. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44.
Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, et al. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene. 2009;28:2513–23.
Katoh Y, Katoh M. Comparative integromics on FAT1, FAT2, FAT3 and FAT4. Int J Mol Med. 2006;18:523–8.
Edris B, Espinosa I, Mühlenberg T, Mikels A, Lee CH, Steigen SE, et al. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol. 2012;227:223–33.
Lu BJ, Wang YQ, Wei XJ, Rong LQ, Wei D, Yan CM, et al. Expression of WNT-5a and ROR2 correlates with disease severity in osteosarcoma. Mol Med Rep. 2012;5:1033–6.
Morioka K, Tanikawa C, Ochi K, Daigo Y, Katagiri T, Kawano H, et al. Orphan receptor tyrosine kinase ROR2 as a potential therapeutic target for osteosarcoma. Cancer Sci. 2009;100:1227–33.
Lara E, Calvanese V, Huidobro C, Fernández AF, Moncada-Pazos A, Obaya AJ, et al. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9:170.
Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, Liu XH. Loss of Wnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol. 2012;18:1328–38.
Daneshmanesh AH, Porwit A, Hojjat-Farsangi M, Jeddi-Tehrani M, Tamm KP, Grandér D, et al. Orphan receptor tyrosine kinases ROR1 and ROR2 in hematological malignancies. Leuk Lymphoma. 2013;54:843–50.
Endo M, Doi R, Nishita M, Minami Y. Ror family receptor tyrosine kinases regulate the maintenance of neural progenitor cells in the developing neocortex. Cell Sci. 2012;125:2017–29.
Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol. 2001;211:161–71.
Saitoh T, Katoh M. FRAT1 and FRAT2, clustered in human chromosome 10q24.1 region, are up-regulated in gastric cancer. Int J Oncol. 2001;19:311–5.
van Amerongen R, Nawijn M, Franca-Koh J, Zevenhoven J, van der Gulden H, Jonkers J, et al. Frat is dispensable for canonical Wnt signaling in mammals. Genes Dev. 2005;19:425–30.
Wang Y, Liu S, Zhu H, Zhang W, Zhang G, Zhou X, et al. FRAT1 overexpression leads to aberrant activation of beta-catenin/TCF pathway in esophageal squamous cell carcinoma. Int J Cancer. 2008;123:561–8.
Zhang Y, Han Y, Zheng R, Yu JH, Miao Y, Wang L, et al. Expression of Frat1 correlates with expression of β-catenin and is associated with a poor clinical outcome in human SCC and AC. Tumour Biol. 2012;33:1437–44.
Wang Y, Hewitt SM, Liu S, Zhou X, Zhu H, Zhou C, et al. Tissue microarray analysis of human FRAT1 expression and its correlation with the subcellular localisation of beta-catenin in ovarian tumours. Br J Cancer. 2006;94:686–91.
Guo G, Mao X, Wang P, Liu B, Zhang X, Jiang X, et al. The expression profile of FRAT1 in human gliomas. Brain Res. 2010;1320:152–8.
Acknowledgments
This study was supported by the Central South University’s fund for independent exploration and innovation of Graduate (No.: 2013zzts336).
Conflict of interest
All authors declared no any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, L., Yang, Z., Zhou, J. et al. The clinical pathological significance of FRAT1 and ROR2 expression in cartilage tumors. Clin Transl Oncol 17, 438–445 (2015). https://doi.org/10.1007/s12094-014-1254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1254-y