Abstract
Objectives
The present study aimed to investigate the expression level of MicroRNA-25 (miR-25) in epithelial ovarian cancer (EOC) tissue, and examine its relationship with clinicopathological factors and prognosis of patients with EOC.
Methods
Expression levels of miR-25 in 86 pairs of EOC tissue and adjacent normal tissue were measured by quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR). The comparison of the expression level of miR-25 between EOC tissue and adjacent normal tissue was performed using the two-sample Student’s t test. The correlation between the expression of miR-25 and clinicopathological characters was assessed with the two-sample Student’s t test. The overall survival was analyzed by log-rank test, and survival curves were plotted according to Kaplan–Meier.
Results
The expression level of miR-25 in EOC tissue was significantly higher than in adjacent normal tissue. The miR-25 expression level was significantly positively correlated with tumor stage, histology, and regional lymph node involvement (P < 0.05). Kaplan–Meier analysis showed that patients with higher levels of miR-25 had significantly poorer survival than those with lower expression of this miRNA in patients, with a 6-year overall survival of 15.96 and 45.89 %, respectively, (P = 0.001). In the multivariate Cox proportional hazards analysis, high miR-25 expression was independently associated with poor survival (P = 0.002; HR = 2.119; 95 % CI = 1.568–3.221).
Conclusion
The increased expression of miR-25 is closely related to poor prognosis of EOC, indicating that miR-25 may serve as a predictive biomarker for the prognosis of EOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy and the second leading cause of cancer-related deaths among women worldwide, and in the majority of cases present with disease that has spread beyond the pelvis [1, 2]. Most cases are not diagnosed until the disease is at an advanced stage. As a result, less than 50 % of patients are alive 5 years after initial diagnosis. Therefore, it is urgent to find new biomarkers, which are able to identify specific patients who may benefit from aggressive therapies and predict the prognosis of epithelial ovarian cancer [3–5].
MicroRNAs (miRNAs) are small, endogenous RNA molecules with 18–25 nucleotides in length [6]. They have been found to be able to regulate gene expression either by translational repression or degradation of mRNA after targeting the 3′UTR [7]. By regulating their target genes, miRNAs are involved in many physiological processes, including cell proliferation, development, differentiation, apoptosis, and others [8]. In particular, accumulating studies have found the aberrant expression of miRNAs in various cancer types and have described the association of miRNA deregulation with the initiation and progression of human cancers [9]. MiR-25 is a member of the miR-106b~25 cluster, which includes miR-106b, miR-93 and miR-25, that is located within intron 13 of the minichromosome maintenance protein 7 (MCM7) gene on chromosome 7q22.1 [10, 11]. Accumulating evidence shows that up-regulation of miR-25 is associated with several types of human malignant solid tumors, including those of the stomach, liver and prostate [12–14]. The study by Zhang et al. [15] showed that miR-25 was highly expressed both in clinical EOC samples and cell lines. However, its association with the clinicopathological features and prognosis in EOC remains unclear. The aim of this study was to examine the expression level of miR-25 in EOC tissues and adjacent non-tumor tissues and explore its associations with clinicopathological characteristics and prognosis.
Materials and methods
Study cohort and samples
The present study was approved by the Research Ethics Committee of Laiwu people’s Hospital, and written informed consent was obtained from all the patients. All specimens were handled and made anonymous according to the ethical and legal standards. Clinicopathological data including age, pathological stage, histology, lymph node metastases status and tumor grade were collected. Patient characteristics were shown in Table 1. None of the patients recruited in this study had undergone preoperative chemotherapy or radiotherapy. The diagnosis in all patients was confirmed each time by microscopic examination of the material obtained during surgery. The duration of follow-up was calculated from the date of surgery to death or last follow-up, and patients were excluded if they had incomplete medical records or inadequate follow-up. For qRT-PCR, 86 pairs of fresh EOC tissues and matched adjacent normal tissues were collected from patients who underwent surgery between August 2006 and July 2012 in Laiwu people’s Hospital. The fresh tissue specimens were collected and immediately placed in liquid nitrogen and then stored at −80 °C until the isolation of RNA.
RNA isolation and qRT-PCR
Total RNA was isolated from frozen specimen by homogenizing tissue in Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The purity and concentration of RNA were determined using NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The differentially expressed amount of the miR-25 was validated in triplicate by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Briefly, 2 μg of RNA was added to RT reaction, and then, the cDNA served as the template for amplification of PCR with sequence-specific primers (Sangon Biotech, Shanghai, China) using SYBR PrimeScript miRNA RT-PCR kit (Takara Biotechnology Co. Ltd, Dalian, China) on the 7500 Real-Time PCR systems (Applied Biosystems, Carlsbad, CA, USA). The PCR cycling profile was denatured at 95 °C for 30 s, followed by 40 cycles of annealing at 95 °C for 5 s, and extension at 60 °C for 34 s. Small nucleolar RNA U6 was used as an internal standard for normalization. The cycle threshold (C T) value was calculated. The \( 2^{{{ - }\Delta {{C}}_{\text{T}} }} \) (ΔC T = C TmiR25 – C TU6 RNA) method was used to quantify relative amount of miR-25.
Statistical analysis
The comparison of the expression level of miR-25 between EOC tissue and adjacent normal tissue was performed using the two-sample Student’s t test. The correlation between the expression of miR-25 and clinicopathological characters was assessed with the two-sample Student’s t test. The overall survival was analyzed by log-rank test, and survival curves were plotted according to Kaplan–Meier. Univariate Cox regression was performed on each clinical covariate to examine its influence on patient survival. Final multivariate models were based on step-wise addition. A Wald statistic of P < 0.05 was used as the criterion for inclusion in final multivariate models. All tests were two-tailed, and results with P < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 13.0 software (Chicago, Ill., USA) and GraphPad Prism 5 (GraphPad Software Inc., CA, USA).
Results
MiR-25 was up-regulated in EOC tissues
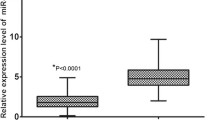
The expression level of miR-25 in the tumor tissues and matched adjacent normal tissues from 86 patients was shown in Fig. 1. The expression level of miR-25 was significantly higher in EOC tissues than in corresponding non-cancerous tissues (6.18 ± 2.31 vs. 2.52 ± 1.97, P < 0.0001).
Comparison of miR-25 expression levels between epithelial ovarian cancer tissue and adjacent normal tissue. Analysis using the two-sample Student’s t test showed that the relative expression levels of miR-25 in the epithelial ovarian cancer tissue were significantly higher than those in adjacent normal tissue (P < 0.0001)
Relation of miR-25 expression level to clinicopathological characteristics
Table 1 presented the results of the correlation analysis between the relative miR-25 expression levels and clinicopathological features of EOC. There was no correlation between the relative miR-25 expression levels and age (P = 0.56) and tumor grade (P = 0.64), but the relative miR-25 expression levels were significantly positively correlated with tumor stage, histology, and regional lymph node involvement The relative miR-25 expression levels were significantly higher in patients with stage III–IV EOC compared with patients with stage I–II EOC (P < 0.001), in patients with serous EOC compared with patients with non-serous EOC (P = 0.002), and in patients with lymph node involvement compared with patients without regional lymph node involvement (P < 0.001).
Expression of miR-25 from patients with EOC in relation to prognosis
To evaluate whether miR-25 expression levels can predict prognosis of patients diagnosed with EOC, we next performed survival analysis. The miR-25 expression levels were classified as high or low in relation to the median value. Kaplan–Meier analysis showed that patients with higher levels of miR-25 had significantly poorer survival than those with lower expression, with a 6-year overall survival of 15.96 and 45.89 %, respectively, (P = 0.001; Fig. 2).
A Cox proportional hazards analysis was used to further evaluate the potential of miR-25 expression as a prognostic biomarker. Univariate survival analyses indicated that miR-25 expression, tumor stage, histology, and regional lymph node involvement were significantly associated with prognosis, while age and tumor grade were not significantly associated with prognosis. In the multivariate Cox proportional hazards analysis, which included age, miR-25 expression level, tumor stage, tumor grade, histology, and regional lymph node involvement, high miR-25 expression was independently associated with poor survival (P = 0.002; HR = 2.119; 95 % CI = 1.568–3.221; Table 2).
Discussion
The discovery that non-coding components of the genome, including miRNA, can contribute to the pathogenesis of cancer has led investigators to contemplate using these molecules to guide clinical decision making [16]. So far, there are more than 1,000 miRNAs annotated by the latest version of miRBase. The expression of miRNAs is remarkably deregulated in ovarian cancer, strongly suggesting that miRNAs are involved in the initiation and progression of this disease [17].
MiR-25 is a member of the miR-106b~25 cluster, which includes miR-106b, miR-93 and miR-25, that is located within intron 13 of the minichromosome maintenance protein 7 (MCM7) gene on chromosome 7q22.1 [10]. Previous studies have shown that the expression of miR-25 was up-regulated significantly in several types of cancers, such as stomach cancer, liver cancer, and prostate cancer [12–14]. In the study by Kim et al. [18], miR-25 was found to be significantly up-regulated in human stomach cancer tissues when compared to adjacent non-neoplastic tissues. The high expression of miR-25 in stomach cancer tissues may be a high risk factor associated with tumor penetration through serosa, lymph node metastasis, distant metastasis, and poor long-term survival in patients undergoing radical resection and adjuvant systemic chemotherapy. In the study by Li et al. [13], more than 50 % of the hepatocellular carcinoma samples showed a greater than twofold increase in the expression of the miR-106b-25 cluster when compared with the corresponding paired non-tumor samples. Knock-down studies for the miR-106b-25 cluster, which includes miR-106b, miR-93 and miR-25, showed that the expression of the cluster was necessary for cell proliferation and for anchorage independent growth. Poliseno et al. [14] found that miR-106b~25 cluster was aberrantly overexpressed in human prostate cancer, correlate with abundance of the miRNA-processing enzyme DICER, and potentiate cellular transformation both in vitro and in vivo. They demonstrated that the intronic miR-106b~25 cluster cooperated with its host gene MCM7 in cellular transformation both in vitro and in vivo. However, investigators also found that the expression of miR-25 was down-regulated in other cancers. For example, Li et al. [19] found that the expression of miR-25 was significantly down-regulated in colon cancer, and miR-25 might suppress the proliferation and migration of colon cancer cells as a tumor suppressor gene in vitro and in vivo. Therefore, we speculate that the function of miR-25 is tissue specific.
Previously, Zhang et al. [15] have found that miR-25 was strongly up-regulated in EOC tissues versus adjacent non-tumor tissues. The expression levels of miR-25 in EOC cell lines were similar with EOC samples compared with the normal ovarian epithelial cells. Taken together, their results demonstrated that miR-25 was highly expressed both in EOC samples and cell lines. However, the clinical significance of miR-25 expression in EOC remains unclear. In the present study, we found that miR-25 expression was proven to be significantly associated with advanced clinical stage, and lymph node metastases, suggesting that miR-25 might be involved in the carcinogenesis and metastasis of EOC. More importantly, we proved that patients with a high expression of miR-25 tended to have shorter survival than patients with lower levels, indicating that high miR-25 level was a marker of poor prognosis for patients with EOC. However, the precise molecular mechanisms behind the altered expression of miR-25 in EOC and its function are not very clear. Zhang et al. [15] found that overexpression of miR-25 in ovarian cancer cells enhanced cell proliferation, whereas down-regulation of miR-25 induced apoptosis. The effects of miR-25 abrogation were partly mediated by the intrinsic apoptosis pathway. Many pro-apoptotic proteins such as Bim, Bax and caspase-3 were up-regulated after transfection. Furthermore, luciferase assays demonstrated that Bim was the direct target of miR-25. Additional studies are needed to more clearly and comprehensively articulate the molecular mechanisms of both the cause and the effects of altered expression of miR-25 in the development and/or progression of EOC.
In conclusion, the present study showed that miR-25 levels were significantly elevated in EOC tissues compared with adjacent normal tissues, and high expression of miR-25 was correlated with poor prognosis for EOC. Therefore, miR-25 may potentially be a putative biomarker for EOC prognosis.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
Haruta S, Furukawa N, Yoshizawa Y, Tsunemi T, Nagai A, Kawaguchi R, et al. Molecular genetics and epidemiology of epithelial ovarian cancer (review). Oncol Rep. 2011;26(6):1347–56.
Bast RC, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15 Suppl 3:274–81.
Reade CJ, Riva JJ, Busse JW, Goldsmith CH, Elit L. Risks and benefits of screening asymptomatic women for ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;130(3):674–81.
Xu L, Cai J, Yang Q, Ding H, Wu L, Li T, et al. Prognostic significance of several biomarkers in epithelial ovarian cancer: a meta-analysis of published studies. J Cancer Res Clin Oncol. 2013;139(8):1257–77.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Cho WC. MicroRNAs in cancer—from research to therapy. Biochim Biophys Acta. 2010;1805(2):209–17.
Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–6.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–69.
Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68(20):8191–4.
Savita U, Karunagaran D. MicroRNA-106b-25 cluster targets beta-TRCP2, increases the expression of Snail and enhances cell migration and invasion in H1299 (non small cell lung cancer) cells. Biochem Biophys Res Commun. 2013;434(4):841–7.
Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, et al. Functional links between clustered microRNAs: suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37(5):1672–81.
Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100(7):1234–42.
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, et al. Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal. 2010;3(117):ra29.
Zhang H, Zuo Z, Lu X, Wang L, Wang H, Zhu Z. MiR-25 regulates apoptosis by targeting Bim in human ovarian cancer. Oncol Rep. 2012;27(2):594–8.
Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93(1):98–104.
Spiliotis J, Halkia E, Roukos DH. Ovarian cancer screening and peritoneal carcinomatosis: standards, ‘omics’ and miRNAs for personalized management. Expert Rev Mol Diagn. 2011;11(5):465–7.
Kim BH, Hong SW, Kim A, Choi SH, Yoon SO. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol. 2013;107(5):505–10.
Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H, et al. MicroRNA-25 functions as a potential tumor suppressor in colon cancer by targeting Smad7. Cancer Lett. 2013;335(1):168–74.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Meng, X., Li, H. et al. MicroRNA-25 expression level is an independent prognostic factor in epithelial ovarian cancer. Clin Transl Oncol 16, 954–958 (2014). https://doi.org/10.1007/s12094-014-1178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-014-1178-6