Abstract
Purpose
T cells are dominant in the immune regulation of malignant pleural effusion (MPE). However, it is unclear about the role of IL-17+ T cells, particularly for IL-17+CD8+ Tc17 cells in antitumor immunity. This retrospective study is aimed at evaluating the prognostic significance of IL-17+ T cells in patients with MPE.
Methods
The frequency of IL-17+CD4+ Th17 and IL-17+CD8+ Tc17 cells in peripheral blood (PB), pleural fluids (PF), and tumor tissues in 24 patients undergoing thoracoscopy was determined by flow cytometry, immunohistochemistry, and ELISA. The association among the different measures was analyzed by Spearman’s correlation tests.
Results
The percentages of PF Th17 and Tc17 cells were significantly higher than those in the PB of MPE patients and healthy controls (p < 0.01). Analysis of Th17 and Tc17 cells in the tumor tissues indicated that the percentages of Th17 and Tc17 cells in the invading tumor edge were significantly higher than those in the non-tumor tissues and intra-tumor regions (p < 0.05). More importantly, the percentages of IL-17+ T cells were associated with prolonged survival of patients with MPE.
Conclusions
Both Th17 and Tc17 cells were involved in the tumor immunity against MPE. Increased frequency of Tc17 cells may serve as a biomarker for the prognosis of patients with MPE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
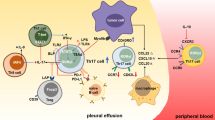
Pleural effusion (PE) is a frequent clinical problem with multiple causes, and the two most common etiologies are tuberculosis (TB) and cancer [1]. Th17 cells are important pro-inflammatory players and can secrete IL-17. Th17 cells participate in antitumor immunity and are involved in the pathogenesis of graft rejection, autoimmune disease, and TB [2, 3]. Previous studies have shown increased numbers of circulating Th17 cells in patients with malignant PE (MPE) and are associated with improved patient survival [4, 5]. A recent study shows IL-17+CD8+ T cells (Tc17) in some patients with inflammatory disease [6]. It is well known that CD8+ T cells are predominant fighters against tumors and that CD8+ T cells are also present in MPE lesions [7]. However, there is no information about the potential role of Tc17 and IL-17 in the pathogenic process of MPE in humans.
This study is aimed at investigating the concentrations of serum IL-17 and at the frequency of Tc17 cells in peripheral blood and tumor tissues in patients with MPE.
Materials and methods subjects
Collection of sample
This study was approved by the Institutional Review Board of the First Affiliated Hospital of China, Three Gorges University, Yi Chang Central People’s Hospital. Written informed consent was obtained from individual subjects. A total of 24 patients with newly diagnosed lung adenocarcinoma and pleura metastasis, including 8 females and 16 males (age 50.1 ± 8.7 years), were recruited at the inpatient service of the First Affiliated Hospital of China, Three Gorges University from 2011 June to 2013 June. Individual patients with lung adenocarcinoma and pleura metastasis were diagnosed, according to computed tomography (CT) scanning and pleural endoscopy as well as histological examination of biopsied pleural tissues. Individual patients were excluded if she/he received any disease-modifying drugs, anticancer therapy, glucocorticoid treatment, had an autoimmune disease, empyema, chest trauma, was pregnant or during the lactating period before recruitment [8]. All patients were subjected to a pleural endoscopy and thoracoscopy, and their pleural effusion (PE) and different pleural tissues (the edge of tumor, intra-tumor, and non-tumor regions) were sampled, followed by pathological examination. Another 24 healthy subjects, including 9 females and 15 males, with a mean age of 46.5 ± 11.3 years, were recruited at the Physical Examination Center of our hospital, and they had no history of any chronic disease and no current infection. Their demographic and clinical data were recorded and are shown in Table 1.
Laboratory tests of PE
The concentrations of PE lactate dehydrogenase (LDH), carcinoembryonic antigen (CEA), total proteins, total numbers of immune cells, and the percentages of lymphocytes, neutrophils, and Mesothelial cells were routinely examined by enzyme colorimetry and chemiluminescence immunoassay using special kits, according the manufacturers’ instructions (Huachenbio, Shanghai, China), and as well as an automatic analyzer (Beckman Coulter, Fullerton, USA).
Processing of samples
Peripheral blood (PB) samples were collected from individual patients within 24-h post-admission. All of the PB and PE samples were centrifuged at 300×g for 5 min, and plasma and PE supernatants were collected and stored at −80 °C for enzyme-linked immunosorbent assay (ELISA). Another portion of the PB samples was used for flow cytometry. One portion of the fresh pleura tissue samples was cut into small pieces and digested with 0.05 % collagenase IV (Sigma-Aldrich, St. Louis, MO) and 0.002 % DNase I (Roche, Basel, Switzerland) at 37 °C for 20 min. The dissociated cells were filtered through a strainer mesh (200 μm) and re-suspended in 20 % fetal calf serum (FBS) RPMI 1640 (Hyclone Company, USA) to isolate the mononuclear cells. Another portion of the pleura tissue samples was fixed in 10 % neutral-buffered formalin and paraffin-embedded for immunohistochemistry.
Flow cytometry
The PB and PE samples as well as the disassociated biopsied tissue cells were subjected to isolation of lymphocytes using the Lymphoprep (Stemcell Techol, Vancouver, Canada) by density-gradient centrifugation, according to the manufacturers’ instruction. The isolated lymphocytes at 2 × 106 were stimulated with 50 ng/ml of phorbol myristate acetate (PMA) and 1.0 μg/ml ionomycin (Sigma) in 10 % FBS RPMI1640 at 37 °C in a humidified atmosphere of 5 % CO2 for 2 h and exposed to 6 μg/ml of Brefeldin A and 2.8 μg/ml of monensin (eBioscience, San Diego, CA) for 4 h. The cells were stained in duplicate with PE-Cy5.5-anti-CD3, FITC-anti-CD4, PE-anti-IL-17, or PE-IgG1 control (eBioscience). The percentages of IL-17+ T cells were determined by flow cytometry on a C6 flow cytometer (BD Accuri Cytometers, Ann Arbor, USA), and the data were analyzed by the Accuri CFlow Plus software (version 1.0.227.4).
Immunohistochemistry
The paraffin-embedded pleural biopsied tissue sections (4 μm) were deparaffinized, rehydrated, and retrieved by boiling with Tris–EDTA (Invitrogen, San Diego, USA) at 121 °C for 3 min. The sections were treated with 3 % H2O2 in methanol at room temperature for 10 min and blocked with 5 % fat-free dry milk in PBS. Subsequently, the tissue sections were incubated overnight with primary antibodies against CD4, CD8, and IL-17 (R&D Systems, Minneapolis, USA) at 4 °C. After being washed with 0.03 % Tween 20 in PBS, the bound antibodies were detected with rabbit antihuman IgG at 37 °C for 1 h and visualized with DAB (Gene, Shanghai, China), followed by counterstaining with Harris hematoxylin (Baso, Zhuhai, China). The percentages of positive cells were calculated at 10 high magnifications (×400) of each section and five sections per sample.
Measurement of IL-17
The concentrations of plasma IL-17 were quantified by ELISA using human IL-17 specific kit, according to the manufacturer’s instruction (eBioscience). The limitation of detection for IL-17 was 0.23 pg/ml.
Statistical analysis
All data were subjected to normality test and expressed as the mean ± standard deviation (SD). The difference between two groups was analyzed by Student’s t test or paired t test. The relationship between two variants was analyzed by Spearman’s correlation test. The survival of each group of patients was estimated by the Kaplan–Meier method, and the difference between two groups was analyzed by the log-rank test. All statistical analyses were performed using the SPSS software (version 13.0, Chicago, IL, USA). A two-tailed P value <0.05 was considered statistically significant.
Results
Increased frequency of Th17 and Tc17 cells in MPE
To determine the importance of IL-17+ T cells in the development of MPE, 24 patients with MPE and 24 healthy controls (HC) were recruited. There was no significant difference in the distribution of age and gender between the patients and HC. Analysis indicated that the concentrations of LDH, CEA, and total proteins were 429.4 ± 102.7 IU/l, 416.5 ± 129.1 μg/l, and 46.1 ± 10.4 g/l, respectively. There were 2.29 ± 0.93 × 109/ml of total cells in the PF, which contained 49.8 ± 6.7 % of lymphocytes, 8.9 ± 2.5 % of neutrophils, and 2.4 ± 0.8 % of mesothelial cells.
Flow cytometry analysis revealed that there was no significant difference in the numbers of PBMCs and in the percentages of CD3+ T cells between the MPE patients and HC (data not shown). The percentages of PB IL-17+CD4+ Th17 and IL-17+CD8+ Tc17 cells in the MPE patients were similar to that of HC, but were significantly lower than those in the PF of MPE patients (p < 0.000 for both, Fig. 1a–e). The percentages of PF IL-17+CD4+ Th17 cells were correlated positively with the percentages of PF IL-17+CD8+ Tc17 cells in the MPE patients (r = 0.867, p < 0.000, Fig. 1f). ELISA analyses indicated that there was no significant difference in the levels of plasma IL-17 between the MPE patients and HC (O. D. values 0.90 ± 0.67 vs. 0.88 ± 0.64, p > 0.05). Collectively, significantly increased IL-17 responses occurred in the PF of MPE patients.
Peripheral blood and pleural fluid samples from individual subjects were stained with fluorescent antibodies, and after lysis of red blood cells, the percentages of Th17 and Tc17 cells were characterized by flow cytometry. The cells were gated on living lymphocytes (A), and the CD3+CD4− and CD3+CD4+ T cells (B) were further analyzed for the frequency of IL-17+CD4+ Th17 and IL-17+CD4− Tc17 cells (C). Data shown are representative charts and expressed as the mean values of individual samples. (D) The percentages of Th17 cells. (E) The percentages of Tc17 cells. (F) The correlation between the percentages of Th17 and Tc17 cells in MPE patients. The horizontal lines indicate the median values of each group.
Distribution of IL-17+ T cells in MPE patients
To evaluate the distribution of IL-17+ T cells in the pleural tissues, we isolated lymphocytes from the different regions of biopsied pleura tissues and stimulated with PM/ionomycin in vitro, followed by characterizing IL-17+ T cells (Fig. 2a, b). First, the percentages of pleura tissue IL-17+CD4+ Th17 and IL-17+CD8+ Tc17 cells were significantly higher than those in the PB of MPE patients (p < 0.05). Second, the percentages of IL-17+CD4+ Th17 and IL-17+CD8+ Tc17 cells in the non-tumor tissues were significantly higher than those in the intra-tumor regions (p < 0.05 for both types of T cells), but were significantly lower than those in the invading tumor edge (p < 0.05 for both types of T cells) in MPE patients. Furthermore, immunohistochemistry analyses revealed that the numbers of CD4+, CD8+, and IL-17+ cells in the invading tumor edges were obviously greater than those in the non-tumor and intra-tumor regions in MPE patients (Fig. 3).
In addition, the percentages of IL-17+CD8+ T cells in the invading tumor edges (23.61 ± 13.75 %), non-tumor region (23.12 ± 14.04 %), and intra-tumor regions (18.15 ± 13.33 %) were significantly higher than those in the PB (12.65 ± 7.97 %) of MPE patients (p < 0.01 for all). Therefore, there was a high frequency of IL-17+ T cells in the invading edges of lung adenocarcinoma in patients with MPE.
Peripheral blood and biopsied pleural tissue samples were obtained from 19 patients with MPE and lymphocytes were isolated from the different regions of biopsied tissues. The percentages of Th17 and Tc17 cells in total CD4+ or CD4− T cells were characterized by flow cytometry. Data are representative charts (A) and expressed as the mean values of percentages of Th17 and Tc17 cells from individual patients (B). The horizontal lines indicate the median values of individual groups.
The biopsied pleural tissue sections were stained by anti-CD4, anti-CD8, and anti-IL-17, respectively, and the immunoreactivity was visualized by DBA (brown color). Data shown are representative images (magnification ×400) from 19 samples.
Increased frequency of Tc17 cells is associated with a better prognosis in the survival of patients with MPE
To determine the importance of IL-17+ T cells, we stratified patients into the high percentage of Tc (IL-17+CD8+ Tc17 cells ≥1.77 %, n = 12) and low percentage Tc groups (IL-17+CD8+ Tc17 <1.77 %, n = 12), according the median value of the percentage of IL-17+CD8+ Tc17 cells. We found that although there was no significant difference in the concentrations of plasma IL-17 between these two groups of patients, the mean survival period in the high percentage Tc17 group of patients was significantly longer than that in the low percentage Tc17 group of patients (15.528 vs. 8.817 months, p < 0.05, Fig. 4a). Further analysis indicated that the mean survival periods of individual patients were correlated positively with the percentages of PF IL-17+CD8+ Tc17 cells in these patients (r = 0.73, p = 0.001, Fig. 4b). Collectively, T cells may participate in antitumor immunity, and the percentages of PF T cells may serve as a biomarker for the prognosis of patients with MPE.
All patients were stratified according to the percentages of Tc17 cells >1.7 % (n = 12 per group), and their survival was estimated (A). Furthermore, the potential correlation between the percentages of Tc17 cells, the survival lengths of these patients (B) or Th17 cells, and the mean survival periods of those patients (C) were analyzed by the Spearman’s rank correlation coefficient.
Discussion
Patients with cancer usually suffer from immunosuppression [9, 10], although malignant tumors are often associated with inflammatory infiltrates [11]. In malignant pleural effusion patients, CD4+ and CD8+ T cells are present in their pleural effusion, and the ratios of CD4+ to CD8+ T cells are similar to that in peripheral blood [12–14]. Previous studies have shown that different subsets of CD4+ T cells, such as Th1, Th2, and Th17 cells, are present in the pleural fluids [15–17]. However, there is little information on IL-17+ CD8+ Tc17 cells in the pleural fluids of malignant pleural effusion patients. In this study, we found that the percentages of Tc17 cells, like Th17 cells, were significantly higher than those in the peripheral blood of malignant pleural effusion patients and healthy controls. In addition, the percentages of both pleural fluid Tc17 and Th17 cells were positively correlated in 4 patients. In parallel, characterization of IL-17+ T cells in the biopsied tumor tissues indicated that Tc17 and Th17 cells were predominantly in the edge of the tumor rather than in the intra-tumor and non-tumor regions. Similarly, immunohistochemistry revealed that obviously higher immunoreactivity was detected in the edge of the tumors. Given that CD8+ T cells are important players of antitumor immunity, the significantly increased numbers of Tc17 cells in the edge of the tumors of malignant pleural effusion patients suggest that Tc17 cells may participate in antitumor immunity.
More importantly, stratification analysis indicated that patients with a higher frequency of Tc17 cells had a longer period of survival and that the percentages of pleural fluid Tc17 cells were correlated positively with the lengths of survival in the malignant pleural effusion patients. In addition, the percentages of pleural fluid Th17 cells were correlated positively with the values of mean survival time in the malignant pleural effusion patients. Apparently, the frequency of pleural fluid Th17 and Tc17 cells may serve as a biomarker for the prognosis of malignant pleural effusion patients. These novel findings suggest that both Th17 and Tc17 cells may work together to defend against the progression of tumors in malignant pleural effusion patients. The increased percentages of Th17 and Tc17 cells may stem from the migration of peripheral blood IL-17+ T cells. Alternatively, the tumor environment may promote the differentiation of activated uncommitted T cells toward IL-17+ direction [18]. Indeed, high levels of IL-6 are commonly detected in the pleural fluids and IL-6 is a crucial inducer of IL-17+ T cell development [19, 20]. We are interested in further investigating the function of Tc17 cells and their development.
In conclusion, our data showed a significantly higher frequency of Th17 and Tc17 cells in the pleural fluids of malignant pleural effusion patients and that Th17 and Tc17 cells predominantly resided in the edge of the tumors, rather than in the intra-tumor and non-tumor areas. More importantly, we found that a higher frequency of pleural fluid Tc17 cells was associated positively with longer survival in malignant pleural effusion patients and that the percentages of pleural fluid Tc17 and Th17 cells were correlated with the lengths of survival of malignant pleural effusion patients. Our novel data suggest that the frequency of pleural fluid Tc17 cells may serve as a biomarker for the prognosis of malignant pleural effusion patients and that both Th17 and Tc17 may participate in antitumor immunity against the progression of tumors in malignant pleural effusion patients. Conceivably, our findings may provide new insights into understanding antitumor immunity in patients with malignant pleural effusion. We recognized that our study had limitations of a small sample size and the lack of functional studies of Tc17 cells. Therefore, further studies of Tc17 cell function in a bigger population are warranted.
References
Hu Y, Zhong Q. Basis of estimating pleural effusion size on CT scan: reasonable grouping of volume percentage. Chest. 2013;143:1839.
Bosch DJ, Muijs CT, Mul VE, Beukema JC, Hospers GA, Burgerhof JG, et al. Impact of neoadjuvant chemoradiotherapy on postoperative course after curative-intent transthoracic esophagectomy in esophageal cancer patients. Ann Surg Oncol. 2013. doi:10.1245/s10434-013-3316-8.
Xu CH, Zhan P, Yu LK, Zhang XW. Diagnostic value of pleural interleukin 17 and carcinoembryonic antigen in lung cancer patients with malignant pleural effusions. Tumour Biol J Int Soc Codev Biol Med. 2013. doi:10.1007/s13277-013-1220-2.
Johnson BE, Kabbinavar F, Fehrenbacher L, Hainsworth J, Kasubhai S, Kressel B, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3926–35.
Yang WB, Ye ZJ, Xiang F, Zhang JC, Zhou Q. Th17/Treg imbalance in malignant pleural effusion. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 2013;33:27–32.
Kumawat AK, Strid H, Tysk C, Bohr J, Hornquist EH. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol. 2013;55:355–64.
Yang WB, Liang QL, Ye ZJ, Niu CM, Ma WL, Xiong XZ, et al. Cell origins and diagnostic accuracy of interleukin 27 in pleural effusions. PLoS ONE. 2012;7:e40450.
Ye ZJ, Zhou Q, Zhang JC, Li X, Wu C, Qin SM, et al. CD39+ regulatory T cells suppress generation and differentiation of Th17 cells in human malignant pleural effusion via a LAP-dependent mechanism. Respir Res. 2011;12:77.
Ghirelli C, Hagemann T. Targeting immunosuppression for cancer therapy. J Clin Investig. 2013;123:1–3.
Kawakami Y, Yaguchi T, Sumimoto H, Kudo-Saito C, Tsukamoto N, Iwata-Kajihara T, et al. Cancer-induced immunosuppressive cascades and their reversal by molecular-targeted therapy. Ann NY Acad Sci. 2013;1284:80–6.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6.
Altomare E, Fallarini S, Biaggi G, Gattoni E, Botta M, Lombardi G. Increased frequency of circulating invariant natural killer T cells in malignant pleural mesothelioma patients. Cancer Biol Ther. 2012;13:702–11.
Penido C, Castro-Faria-Neto HC, Larangeira AP, Rosas EC, Ribeiro-dos-Santos R, Bozza PT, et al. The role of gammadelta T lymphocytes in lipopolysaccharide-induced eosinophil accumulation into the mouse pleural cavity. J Immunol. 1997;159:853–60.
Suzuki E, Kapoor V, Cheung HK, Ling LE, DeLong PA, Kaiser LR, et al. Soluble type II transforming growth factor-beta receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clinical cancer research: an official journal of the American Association for. Cancer Res. 2004;10:5907–18.
Larypoor M, Bayat M, Zuhair MH, Akhavan Sepahy A, Amanlou M. Evaluation of the number of CD4(+) CD25(+) FoxP3(+) Treg cells in normal mice exposed to AFB1 and treated with aged garlic extract. Cell J. 2013;15:37–44.
Richert L, Hue S, Hocini H, Raimbault M, Lacabaratz C, Surenaud M, et al. Cytokine and gene transcription profiles of immune responses elicited by HIV lipopeptide vaccine in HIV-negative volunteers. AIDS (London, England). 2013;27:1421–31.
Zhang C, Gui L, Xu Y, Wu T, Liu D. Preventive effects of andrographolide on the development of diabetes in autoimmune diabetic NOD mice by inducing immune tolerance. Int Immunopharmacol. 2013;16:451–6.
Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–54.
Jain A, Pandey N, Garg RK, Kumar R. IL-17 level in patients with Dengue virus infection and its association with severity of illness. J Clin Immunol. 2013;33:613–8.
Li X, Zhou Q, Yang WB, Xiong XZ, Du RH, Zhang JC. Pleural mesothelial cells promote expansion of IL-17-producing CD8+ T cells in tuberculous pleural effusion. J Clin Immunol. 2013;33:775–87.
Acknowledgments
The authors would like to thank the doctors and nurses at the Respiratory Department of Yichang Central People’s Hospital for their assistance in the collection of the samples. The authors also thank H Yi, L Ai, YF Liu, and D Zhang at Yichang Central Hospital for their technical assistance. This study was supported by Hubei Province Science and Technology Plan, the Natural Science Foundation of Hubei Province, and the Education Department of Hubei Province Natural Science Research Project. The experiment was performed at the central laboratory of Yichang Central People’s Hospital and China Three Gorges University. We also thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, Y., Chen, S.X., Gao, B.A. et al. Cell origins and significance of IL-17 in malignant pleural effusion. Clin Transl Oncol 16, 807–813 (2014). https://doi.org/10.1007/s12094-013-1152-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1152-8