Abstract
Objective
Since brain metastases (BM) is often accompanied by edema, and endostatin (ES) can prevent tumor tissue edema, we investigated the therapeutic effects of ES combined with radiotherapy in the treatment of BM of NSCLC. We also determined the patients who are suitable for this therapy.
Methods
Eighty patients with BM of NSCLC were randomly divided into combination group and radiotherapy alone group. The primary endpoint was overall response rate, and secondary endpoints were overall survival time, cerebral edema index and adverse reactions. These were observed and the expressions of vascular endothelial growth factor receptor 2 (VEGFR2) protein and KDR gene in primary lesions were detected with immunohistochemical method and fluorescence in situ hybridization.
Results
Compared with radiotherapy alone, brain edema was significantly reduced in the ES group (P = 0.003) without marked adverse reactions. For the overall response rate, there was no statistical significant difference between the two groups (control, 90 % vs. ES, 75 %, P = 0.07), but there was statistical significance in the patients with positive VEGFR2 (93 vs. 67.7 %, P = 0.012) or positive KDR gene (94.4 vs. 47.3 %, P = 0.002). In overall survival time, there was no statistical significance in the two groups (P = 0.35), in the tumors with positive VEGFR2 (P = 0.109) or with positive KDR gene (P = 0.147).
Conclusion
Compared with radiotherapy alone, ES combined with radiotherapy can reduce brain edema in NSCLC patients with BM and obtain better short-term response rate in tumors with positive VEGFR2 or positive KDR gene, but does not improve the overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BM) is a common complication that occur in about 20–40 % of patients with malignant tumor, the incidence of BM is about 40 % in non-small cell lung cancer (NSCLC) with a poor prognosis and a median survival of 1–4 months [1]. There are many therapeutic methods for BM, but whole brain radiation therapy (WBRT) is the most common method for the treatment of BM [2]. However, because of potential complications that result from high-dose radiation, the dose of WBRT is limited, and BM is often accompanied by brain edema, so how to use WBRT to control tumor and relieve brain edema are difficult problems in clinical practice.
Endostatin (ES), an angiogenesis inhibitor, has been shown to relieve brain edema and exhibit radiation sensitizing properties. Many preclinical studies have indicated that ES can improve the therapeutic effects of radiotherapy for a variety of malignant tumors and enhance the radiosensitivity of the tumor [3]. We have also observed that ES combined with radiotherapy has better short-term response rate and local control rate in the treatment of NSCLC [4]. In addition, ES can prevent vascular leakage, relieving tumor tissue edema and decreasing interstitial pressure [5]. Therefore, we investigated the therapeutic effects of recombinant human endostatin (RHES) combined with radiotherapy in the treatment of brain metastasis.
Materials and methods
Research design
This study was a randomized, controlled and open clinical phase II (NCT01410370). Patients were randomly assigned to RHES combined with radiotherapy (combination group) and radiotherapy alone. The primary endpoint of the study was short-term efficacy; secondary endpoints were overall survival, incidence of adverse events and cerebral edema after completion of treatment.
Inclusion criteria, exclusion criteria and exit criteria
Inclusion criteria
Inclusion criteria were: patients with single or multiple metastasis tumors of brain and received or not received the treatment for NSCLC; without neurological and (or) psychiatric symptoms; aged 75 years and under; KPS score ≥40; a probability of survival time ≥3 months; measurable lesions according to RECIST standard.
Exclusion criteria
Exclusion criteria were: pregnant or breast-feeding women; patients having severe heart disease or abnormal ECG; evidence of bleeding diathesis or serious infection; uncontrolled mental and nervous disorders.
Exit criteria
Exit criteria were: patients having intolerable toxicity or severe bleeding; receiving other therapies during the clinical research; withdrawing from the clinical research and with failure to follow-up.
Treatment
Radiotherapy
In two groups, WBRT was performed with ONCOR EXPRESS linear accelerator (from Siemens, Germany) with isocenter irradiation of 6MV-X, 3 Gy/fraction, once-daily, five fractions/week for a total of ten fractions; for a single lesion, tumor boost was done with 3D-CRT or IMRT to 10 Gy at 2 Gy/fraction.
RHES treatment
RHES (endostar) was purchased from Xiansheng Pharmaceutical Co., Ltd. Intravenous RHES was given according to 7.5 mg/m2/day during radiotherapy.
VEGFR2 immunohistostaining (IHC)
IHC was performed in the tissues of primary NSCLC which obtained from all 80 patients. Sections were washing, incubation and cooling. Primary antibody of VEGFR2 (from Anbobio, USA) was incubated with the sections at 4 °C overnight followed by PBS washing. Biotinylated secondary antibody was incubated with the sections for 1 h before washing. We followed the assessment criteria to grade the number of immunopositive cells [6].
KDR gene amplification (FISH)
KDR gene amplification of primary NSCLC was performed in all 80 patients. Sections were baked at 70 °C for 10 min, underwent deparaffinization with xylene and washed with deionized water. Pepsin was added followed by addition of 5 μl of FISH hybridization containing target gene, control gene and hybridization solution (1:1:3, from Empire Genomics, USA) and mounted. Assessment criteria for FISH [7].
Observational items
Short-term therapeutic effects
After treatment, the therapeutic effects were evaluated according to RECIST (1.0) standards including complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and good response rate (RR) = CR + PR.
Overall survival time
Overall survival was calculated from the day of randomization until the last follow-up or death, but patients lost to follow-up were censored at the time of last contact date.
Edema index (EI) [8]
According to cranial MRI, the length, width and height of tumors were determined on the enhanced T1WI and brain edema was determined on the axial images of FRFSE-T2WI. The volumes (V) of tumor and brain edema were calculated based on the following formula: V = (4/3)π × abc, and EI = V (edema + tumor)/V (tumor). EI = 1 was regarded as no edema, 1 < EI ≤ 1.5 as mild edema, 1.5 < EI ≤ 2 as moderate edema and EI > 2 as severe edema.
Adverse reactions
Adverse reactions were evaluated according to RTOG adverse events grading criteria.
Statistical Methods
With the response rate of radiotherapy alone at 70 % and the hypothesized response rate at 95 %, we have 80 % power to detect a difference between the experimental group and control group at a P < 0.05. Sample size for each group required 40 patients. Treatment was performed with SPSS17.0 software. Comparisons of enumeration data and post-treatment reactions between both groups were performed with χ2 test. Measurement data were expressed as \( \bar{\varvec{x}} \pm {\text{s}} \) and t test was used in the comparisons of means between groups. Survival rate was plotted using Kaplan–Meier method and Log-rank test was used in the comparison of survival rate between both groups. Statistical significance was established at P < 0.05.
Results
Patient characteristics
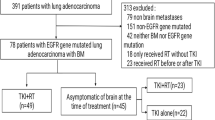
Eighty eligible patients who were diagnosed with BM by MRI and had primary NSCLC were enrolled and randomized between January 2011 and January 2013. The clinical data were similar in both groups (Table 1).
KDR gene amplification and VEGFR2 expression in primary NSCLC
KDR gene located in cell nuclei emitted red fluorescence signals and the centromere in chromosome 4 located in cell nuclei emitted green fluorescence signals (control) (Fig. 1A). VEGFR2 expression was mainly in cytoplasm and on cell membrane (Fig. 1B). There was a significant correlation between IHC results and FISH results (r = 0.282, P = 0.01) (Fig. 1C).The relations between them and clinicopathologic factors are shown in Table 2.
KDR gene amplification and VEGFR2 protein expression in lung cancer tissue. a KDR gene amplification in lung cancer. a Positive amplification, b negative amplification. Red signals are the target genes and green signals are the centromeres in chromosome 4 (×1,000). b VEGFR2 expression is mainly located in cytoplasm and on cell membrane in brown-yellow or brown-black staining. c Pulmonary adenocarcinoma (×200), d pulmonary squamous cell carcinoma (×200). c Comparison of VEGFR2 protein both on cell membrane and in cytoplasm between the patients with positive FISH and the patients with negative FISH. The box plots indicate VEGFR2 protein expression both on cell membrane and in cytoplasm in the patients with positive FISH and the patients with negative patients
Short-term response rates in combination and radiotherapy alone groups
In short-term response rate, there was no statistical significance between the two randomized cohorts (90 % in the combined vs. 75 % in the radiotherapy alone group, χ 2 = 3.11, P = 0.07), but this was significantly increased in the patients with positive VEGFR2 (n = 61, 93 vs. 67.7 %, χ 2 = 6.31, P = 0.012) or in the patients with positive KDR gene (n = 37, 94.4 vs. 47.3 %, χ 2 = 9.8, P = 0.002) between combination and radiotherapy alone groups.
Edema comparison between combination group and radiotherapy alone group after treatment
There was a statistical difference in EI after treatment between combination group and radiotherapy alone group in total population ( t = 4.9, P = 0.000, Fig. 2a1), VEGFR2 positive population ( t = 4.17, P = 0.000, Fig. 2b1) and KDR gene positive population ( t = 3.15, P = 0.003, Fig. 2c1), respectively.
a1 Comparison of edema index before and after treatment between combination and radiotherapy alone groups in total population (n = 80), dP < 0.05 vs. c (t = 5.67), dP < 0.05 vs. b (t = 4.9). a2 Survival curves in combination and radiotherapy alone groups in total population (n = 80, P = 0.35, HP = 0.777, 95 % CI 0.25–1.30). b1 Comparison of edema index before and after treatment between combination and radiotherapy alone groups in the patients with positive VEGFR2 (n = 61), dP < 0.05 vs. c (t = 5.8), dP < 0.05 vs. b (t = 4.17). b2 Survival curves in combination and radiotherapy alone groups in the patients with positive VEGFR2 (n = 61, P = 0.109, HP = 0.875, 95 % CI 0.40–1.34). c1 Comparison of edema index before and after treatment between combination and radiotherapy alone groups in the patients with positive KDR gene (n = 37), dP < 0.05 vs. c (t = 5.64), dP < 0.05 vs. b (t = 3.15). c2 Survival curves in combination and radiotherapy alone groups in the patients
Overall survival time in the combination and radiotherapy alone groups
About overall survival time, this study showed no statistical significance between combination group and radiotherapy alone group in total population (P = 0.35, Fig. 2a2), VEGFR2 positive population (P = 0.109, Fig. 2b2) and KDR gene positive population (P = 0.147, Fig. 2c2), respectively.
Adverse reactions
The most adverse reaction was granulopenia. There was no bleeding or cardiotoxic reaction seen. There were no statistical differences in adverse reactions between combination group and radiotherapy alone groups (Table 3).
Discussion
Many studies have indicated that ES has efficacy in cancer patients [9]. ES, a small molecule protein with multi-target effects, has anti-angiogenic effects mainly through phosphorylation of tyrosine kinase of VEGFR2 (KDR/Flk-1) on endothelial cells [10]. Human VEGFR2, also known as kinase insert domain receptor (KDR), a kind of transmembrane protein kinase receptor, was first separated by Terman in 1991 [11]. VEGFR2 is mainly distributed in vascular endothelial cells, hematopoietic stem cells and macrophages. Recent studies show that VEGFR2 is expressed in malignant tumor cells such as NSCLC [12] and breast cancer [13]. Ghosh Sriparna et al. [14] found that there was positive VEGFR2 mainly located in cytoplasm in 10 % of lung squamous cell carcinoma and 23 % of lung adenocarcinoma. The current study indicated that there was VEGFR2 expression located both in cytoplasm and on cell membrane with a positive rate of 76.2 % (61/80) which is higher than that in other studies, due to the fact that patients with advanced NSCLC were enrolled. Stratified analysis indicated that the positive rate of VEGFR2 was not associated with sex (P = 0.52), pathological types (P = 0.44) and number or location of metastasis (P = 0.43), but was correlated with brain edema (P = 0.000) and primary NSCLC control rate (P = 0.003), suggesting that positive expression of VEGFR2 may be related to the degree of tumor aggressiveness.
Is VEGFR2 expression related to brain edema caused by brain metastases? Kodack et al. [15] found that brain edema caused by brain metastases was mainly related to VEGFR2. After combining with external VEGF, VEGFR2 on tumor cell affects the receptors on tumor vascular endothelial cells through VEGF autocrine loop, leading to increased vascular permeability and leakage. VEGF-VEGFR2 pathway in tumor and endothelial cells is a main reason of brain edema caused by BM [16].
So, we use ES combined with radiotherapy to treat BM and the brain edema effects were evaluated by EI. In this study, compared with radiotherapy alone group, brain edema was significantly reduced (t = 5.81, P = 0.000) and short-term therapeutic effects were significantly improved (χ2 = 6.31, P = 0.012) in the patients with positive VEGFR2 in the combination group. Yang Fei et al. [17] found that there was KDR gene amplification in 32 % of NSCLC tumor tissue and the survival time was shortened in these patients. Our study showed a KDR-amplification rate of 46.2 % (37/80). Stratified analysis demonstrated that KDR-positive rate was not associated with sex (P = 0.61), pathological types (P = 0.26) and number and location of metastasis (P = 0.82), but was correlated with brain edema (P = 0.03) and primary NSCLC control (P = 0.000). Patients with positive VEGFR2, compared with radiotherapy alone group, had brain edema significantly ameliorated (t = 6.49, P = 0.000) and short-term therapeutic effects were significantly improved (χ2 = 9.8, P = 0.002). Although many factors such as therapeutic schedule, pathological types and differentiation affect the overall survival time, this study indicated that we found no difference in overall survival times in the two treatment groups, nor any of the patient subsets.
In summary, RHES combined with radiotherapy can relieve brain edema in the patients with BM of NSCLC and obtain better short-term response rate in the patients with positive VEGFR2 or KDR gene, but fails to significantly improve the overall survival. Therefore, RHES combined with radiotherapy may be suitable for the patients with positive VEGFR2 or/and KDR gene amplification.
References
Lemke DM. Epidemiology, diagnosis, and treatment of patients with metastatic cancer and high-grade gliomas of the central nervous system. J Infus Nurs. 2004;27(4):263–9.
Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, Morris RE, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncology. 2010;96(1):17–32.
Xiao-dong J, Peng D, Jin W, Da-an S, Jin-ming Y. The inhibitory effects of radiotherapy combined with weekly dosage endostatin on the growth of transplanted human lung cancer cell line A549 in nude mice. J. Lung Cancer. 2011;72:165–71.
Jiang XD, Dai P, Wu J, Song DA, Yu JM. Effect of recombinant human endostatin on radiosensitivity in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(4):1272–7.
Jain Rakesh K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89(12):2637–45.
Johansson I, Aaltonen KE, Ebbesson A, Grabau D, Wigerup C, Hedenfalk I, et al. Increased gene copy number of KIT and VEGFR2 at 4q12 in primary breast cancer is related to an aggressive phenotype and impaired prognosis. Genes Chromosom Cancer. 2012;51:375–83.
Inamura T, Nishio S, Takeshita I, Fujiwara S, Fukui M. Peritumoral brain edema in meningiomas influence of vascular supply on its development. Neurosurgery. 1992;32(2):179–85.
Wenyin Shi, Christian Teschendorf, Nicholas Muzyczka, Siemann Dietmar W. Gene therapy delivery of endostatin enhances the treatment efficacy of radiation. Radiother Oncol. 2003;66(1):1–9.
Ling Yun, Yang Yong, Lu Na, You Qi-dong, Wang Sen, Gao Ying, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361(1):79–84.
Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB, et al. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6(9):1677–83.
Donnem T, Al-Shibli K, Andersen S, Al-Saad S, Busund LT, Bremnes RM, et al. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer. 2010;116:4318–25.
Seto T, Higashiyama M, Funai H, Imamura F, Uematsu K, Seki N, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage 1 non-small-cell lung cancer. Lung Cancer. 2006;53:91–6.
Ghosh Sriparna, Zerkowski Maciej P, Molinaro Annette M, Rimm David L, Camp Robert L, et al. High levels of vascular endothelial growth factor and its receptors (VEFR-1, VEGFR2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. 2008;39(12):1835–43.
Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AMMJ, Song Youngchul. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci USA. 2012;109(45):E3119–27.
Chatterjee S, Heukamp LC, Siobal M, Schöttle J, Wieczorek C, Peifer M, et al. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123(4):1732–40.
Yang F, Tang X, Riquelme E, Behrens C, Nilsson MB, Giri U, et al. Increased VEGFR2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res. 2011;71(16):5512–21.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81272558), Research Fund from Ministry of Health (W201210), Fund from China International Medical Communication (CIMF-F-H001-250) and the National Natural Science Foundation of Jiangsu Province (BK2012661).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, X., Ding, M., Qiao, Y. et al. Recombinant human endostatin combined with radiotherapy in the treatment of brain metastases of non-small cell lung cancer. Clin Transl Oncol 16, 630–636 (2014). https://doi.org/10.1007/s12094-013-1129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1129-7