Abstract
The annual incidence of neuroendocrine tumours in the Caucasian population ranges from 2.5 to 5 new cases per 100,000 inhabitants. Gastroenteropancreatic neuroendocrine tumours is a family of neoplasms widely variable in terms of anatomical location, hormone composition, clinical syndromes they cause and in their biological behaviour. This high complexity and clinical heterogeneity, together with the known difficulty of predicting their behaviour from their pathological features, are reflected in the many classifications that have been developed over the years in this field. This article reviews the main tissue and clinical biomarkers and makes recommendations for their use in medical practice. This document represents a consensus reached jointly by the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumours (NETs) are a group of cancers of great clinical, biochemical and biological heterogeneity. Although they share a number of common histopathological features, they arise from neuroendocrine cells in diverse anatomical sites, including ganglia and paraganglia, endocrine glands (pituitary, parathyroid, thyroid, adrenal, and pancreas), skin and numerous organs containing dispersed cells (digestive tract, biliary tract, lung and bronchi, thymus, urogenital system). Also, unlike other solid tumours, they can synthesise and excrete various polypeptide hormones that cause specific clinical syndromes in 20–30 % of cases, classified as “functioning tumours”. However, most are clinically silent until late stages of the disease [1, 2].
Although NETs have traditionally been regarded as indolent tumours, their clinical behaviour is often unpredictable and can sometimes be very aggressive (e.g. poorly differentiated carcinomas and/or those tumours with high proliferative index). The wide range of classifications proposed for these tumours, based on their embryological or anatomical origin, morphological and histological features, biochemical profile and/or clinical behaviour, clearly illustrates their high complexity and diverse nature. All this underscores the need to elaborate consensus guidelines to help standardise the criteria for diagnosing and treating this neoplastic disease, particularly since its relatively low incidence makes clinical management highly variable as it is generally based on low evidence levels.
In this context, the aim of this document was to offer clear, concise guidelines to provide the clinician with practical recommendations on using tissue and serum biomarkers in the diagnosis and treatment of neuroendocrine tumours of gastroenteropancreatic origin (GEP-NETs). These clinical practice guidelines have been elaborated by various specialists in medical oncology and pathology as a joint initiative of the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP).
Epidemiology of NETs of the digestive tract and pancreas
The incidence of NETs in the Caucasian population ranges from 2.5 to 5 new cases per 100,000 inhabitants annually [3, 4]. This incidence has risen considerably in the past few decades, probably due to major advances in diagnostic techniques and greater clinical awareness. The recent development of new effective drugs in this context has certainly contributed to it [5].
About two-thirds of NETs are of gastrointestinal or pancreatic origin (GEP-NETs), and among these the most common site of origin is the small intestine. Although GEP-NETs represent only 2 % of all gastrointestinal tumours, the fact that they have a better prognosis than epithelial cancers makes their relative prevalence much higher, being the second most prevalent gastrointestinal malignancy after colorectal adenocarcinoma. These tumours are diagnosed at a younger age than carcinomas, usually during the fifth decade. NETs can be sporadic or may develop in the context of various syndromes entailing hereditary predisposition, such as multiple endocrine neoplasia type 1 (MEN-1) or von Hippel-Lindau (VHL) disease. In this case, age at diagnosis may be 15 years younger.
According to data from the United States Surveillance, Epidemiology and End Results (SEER) population registry, 49 % of GEP-NETs are diagnosed at localised stages, whereas 27 % display metastatic disease at presentation (64 % pancreatic vs. 30 % ileal; 50 % poorly differentiated tumours vs. 21 % well-differentiated tumours) [3, 4]. These numbers, however, vary greatly from one registry to another, and the overall incidence of metastasis at diagnosis is generally far higher in European databases (44–73 %). Nevertheless, the incidence may be overestimated due to the inherent bias caused by the high number of advanced stages at referral centres, which are the main source of information for most of these registries [6, 7].
According to SEER data, the 5-year overall survival rate is 60–70 %, with the best prognosis for rectal tumours (88 %) and worst for pancreatic ones (37 %). Again, these numbers differ from those seen in our setting, although European registries are generally hospital-based rather than population-based. Also, the prognosis of NETs depends on many factors, the most important being the primary tumour site, the stage of disease spread, the grade of histopathological differentiation and the cellular proliferative index of the tumour. The distribution of these variables in each series may therefore have a considerable influence on the survival rates reported.
Pathology issues
Pathology-based classification
NETs are uncommon heterogeneous neoplasms characterised by their ability to secrete glycopeptide hormones and active amines. Of the 15 types of neuroendocrine cells described in the gastroenteropancreatic tract, 9 have been identified in NETs. GEP-NETs vary considerably in their hormone composition, in the syndromes they cause, and in their biological behaviour. This high complexity and biological heterogeneity, together with the acknowledged difficulty of predicting their behaviour from their pathological features, are reflected in the various classifications of GEP-NETs developed during the last century [8, 9].
Initially, Oberndorfer termed these tumours “carcinoid” in 1907. Then Williams and Sandler, in 1963, classified them based on their embryological origin and anatomical site in foregut, midgut or hindgut tumours. Last, a new classification by Capella, Heitz, Solcia et al. in the 1990s [10, 11] formed the basis for the general classification of GEP-NETs, and the particular pancreatic NETs, respectively, published by the World Health Organisation (WHO) in 2000 and 2004.
The need for standardised systems of stratification and management of patients affected by these neoplasms led the European Neuroendocrine Tumour Society (ENETS) to elaborate some guidelines for the diagnosis and treatment of these tumours. These guidelines proposed for the first time the TNM classification for NETs, which are specific to each organ or site of origin, some of which differ somewhat from the TNM classification subsequently suggested by the American Joint Committee on Cancer (AJCC) (i.e. pancreas or appendix) (Table 1). ENETS also proposed a standardised grading system based on Ki-67 proliferative index or mitotic index, by which GEP-NETs are classified into three grades (Table 2) [12].
When a sample shows morphological features of a NET-like tumour, the first step is to demonstrate its neuroendocrine nature using chromogranin A or synaptophysin immunostaining. Then, the proliferative activity should be established either by counting mitoses in 50 high-power fields (HPFs), and selecting the highest areas, or “hot spot” areas, i.e., 10 of the most active HPFs (mitoses per 10 HPFs) or, preferably, by determining the Ki-67 proliferative index using the MIB-1 antibody on 2,000 cells in areas of greatest immunostaining (e.g. counting 20 sets of 100 cells) (Table 2). This grading system has been incorporated into the latest WHO classification, dated 2010, which is the currently used version (Table 3).
It should be noted that some controversy exists concerning on which tumours are considered as “poorly differentiated neuroendocrine carcinomas” (NEC). In the current classification, this category includes both NETs with well-differentiated morphology that meet G3 proliferation criteria (mitosis and Ki-67) and small-cell and large-cell NECs. Some authors believe that these two tumour subtypes differ in their biology and clinical behaviour. In this respect, molecular studies have shown that the profile of NETs differs completely from that found in NECs [13] and that whereas a G1 or G2 NET may progress to a G3 NET, progression to a NEC is rare.
Essential neuroendocrine tissue markers
The demonstration of neuroendocrine differentiation using immunohistochemical markers is a great help in the diagnosis of NETs. The recommended markers are chromogranin A and synaptophysin, which should be used in the great majority of NETs, including biopsies of metastatic tumours (Table 4) [12, 14]. However, it should be noted that there is disagreement as to whether they should be used in all cases or only in those with unusual or poorly differentiated morphological features [12]. The use of other neuroendocrine markers such as chromogranin B, CD56 (N-CAM) or CD57 (LEU7) is not generally recommended, given their low levels of sensitivity and/or specificity [14], although they are optional in cases in which one of the main markers (chromogranin A or synaptophysin) is negative.
The proliferation rate in NETs provides relevant prognostic information, and most grading systems that divide NETs into low, intermediate or high grades are based on this parameter [15–17]. The cell proliferation rate can be assessed by counting mitotic figures (usually as mitoses per 10 HPFs or per 2 mm2), or by estimating what percentage of nuclei are stained with the cellular proliferation marker Ki-67. The WHO classification of lung and thymus NETs is based on counting mitoses, whereas the system for NETs of the gastrointestinal tract proposed by ENETS, and adopted in the new WHO classification, recommends using either the mitotic count or the Ki-67 proliferation rate [14–16]. Quantification by Ki-67 has become an essential component of the pathology report on NETs and is included in the protocols issued by the College of American Pathologists, with cut-off points of 2 and 20 % for grade 2 and grade 3, respectively [6, 14].
Quantification of the proliferative index using the Ki-67 antibody should be done as precisely as possible in the areas of greatest proliferative activity (“hot spots”) as the choice of these areas is the most important source of variation between different observers. Another source of error is the inclusion of non-neoplastic positive cells, such as lymphocytes and stromal elements, in the quantification [18]. Once these potential problems have been considered, the choice of quantification method to be used must be made. The simplest method is a rough “eyeballed” estimate of individual cells, although most authors regard this as highly subjective and imprecise [18]. Automated systems have also been proposed, although they also have some disadvantages, as they can count non-neoplastic elements or haemosiderin, besides that they do not solve the problem of choosing which area of the tumour to be counted. Moreover, the automated systems are expensive and not always available for daily clinical practice. The most effective method is a cell-by-cell count, either on the computer screen or on a print-out of the image captured, of 2,000 cells in hot spot areas that show highest immunostaining. This is a highly reproducible fast option (estimated time, 6 min) that only requires a digital camera connected to a computer [18, 19].
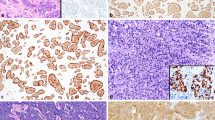
In summary, Ki-67 provides a precise, reliable method for quantifying the proliferation rate, provided painstaking methods are used to supplement or replace the counting of mitotic figures (Fig. 1).
Illustrative examples of gastroenteropancreatic neuroendocrine tumours: G1 well-differentiated neuroendocrine tumour, haematoxylin and eosin (a) and Ki-67-MIB-1 (b); G2 well-differentiated neuroendocrine tumour, haematoxylin and eosin (c) and Ki-67-MIB-1 (d); G3 neuroendocrine carcinoma, haematoxylin and eosin (e, g) and Ki-67-MIB-1 (f, h); mixed adenoneuroendocrine carcinoma, haematoxylin and eosin (I) and chromogranin A (j)
Optional neuroendocrine tissue markers
The use of additional neuroendocrine markers, such as chromogranin B, CD56 (N-CAM) and CD57 (LEU7), is advisable in cases of NETs with atypical morphology, although it should be noted that these are not specific markers. Neuron-specific enolase (NSE) is not advisable in any case. Most well-differentiated NETs express cytokeratins 8 and 18 (CK8 and CK18) and are detected by the Cam 5.2 antibody. Some tumours are positive for CK7 and CK20. Although 70 % of NETs are p53-positive by gene mutation, p53 staining provides no further information [14]. Annenkov et al. [20] used alpha-methylacyl-coenzyme A racemase to distinguish grade 1 gastric NETs (negative) from grade 2 NETs and carcinomas, which are often positive. As regarding the cellular proliferation rate, some authors have shown geminin to be a more precise marker of cell proliferation index than Ki-67. Geminin expression is confined to S phase, G2 and early M phase of the cell cycle. In pancreatic NETs the geminin expression level was associated with disease-free survival after curative resection and was a prognostic predictor [21].
Peptide hormones and amines
Staining with peptide hormones, such as insulin or glucagon, should only be used in special cases, such as MEN-1 with multiple pancreatic tumours in which the functionality of a tumour needs to be demonstrated. In other NETs, functionality is defined by symptoms and serology [14].
Markers used for differential diagnosis
Given the different therapeutic strategies for metastatic NETs of pancreatic or intestinal origin, immunohistochemistry can help identify the organ in which the primary tumour is located when it is not clinically obvious. Testing positive for TTF-1, CK7 or ASH1 suggests a bronchopulmonary origin, although some gastrointestinal NETs may also be positive. Expression of PDX-1 and CDX-2 points to a pancreatic and intestinal site, but these markers are not pathognomonic, as some pulmonary NETs express CDX-2. PDX-1 and ISI-1 increase the likelihood of a pancreatic origin, but do not exclude it if negative. It should be stressed that cadherin 17 (CDH17) is more specific for gastrointestinal origin than CDX-2 [22].
It is better to use antibody panels, rather than just one or two markers, because of their lack of specificity. Chan et al. [23] proposed an immunohistochemical panel including CDX-2, PDX-1, TTF-1 and CK7 to distinguish between gastrointestinal, pancreatic and bronchopulmonary primary tumours. Some colorectal NETs are negative for all markers [14, 24].
Markers of diagnostic and prognostic interest
Haematopoietic stem cell markers such as CD117, PAX-5 and CD34 are positive in 54, 18 and 8 %, respectively. CD117 is a poor prognostic marker associated with vascular invasion. Testing positive for CD117 is not associated with activating mutations in the c-KIT gene, which would explain the inefficacy of imatinib in treating these tumours [22]. In pancreatic tumours other markers of aggressiveness are CK19, cyclooxygenase-2, p27, and CD99 [22, 25].
Fifteen per cent of NETs show a CpG island methylator phenotype (CIMP) and microsatellite instability (MSI). These tumours are associated with a better prognosis and do not express CD117, hMLH1 or hMSH2. They tend to be large-cell tumours with peritumoral lymphoid collections and no vascular invasion [22]. Markers associated with therapeutic response include somatostatin receptor subtypes, MGMT, VEGFR, pIGFR and members of the mTOR pathway. However, the relationship between immunostaining and therapeutic response is currently undergoing validation. High expression levels of AKT phosphorylated at Ser473 and ribosomal protein S6 phosphorylated at Ser240 and Ser244 are associated with short time to progression in patients treated with somatostatin analogues [26, 27].
The NOTCH1 signalling pathway influences the growth and differentiation of gastrointestinal, pancreatic and pulmonary NETs, acting as a tumour suppressor gene. NOTCH1 gene and protein expression are variables dependent on the site of the primary tumour in gastrointestinal NETs, being 100 % positive in rectal tumours, 34 % positive in pancreatic tumours and negative in ileal tumours [28]. The NOTCH1 target, HES1, is expressed in 64 % of rectal NETs and 10 % of pancreatic NETs, and is negative in ileal tumours.
It is important to stress that the PAX-8R1 antibody is not a pancreatic neuroendocrine marker. Studies describing a positive antigen–antibody reaction with PAX-8 used a polyclonal antibody that possesses cross-reactivity with the N-terminal region of PAX-5 and PAX-6 [29].
Sample types, pre-test and test factors, internal and external controls
Sample types
The types of samples depend on the procedure performed to obtain them. They may come from small biopsies, obtained by endoscopy, endoscopic ultrasound or fine-needle aspiration or from resection specimens.
Pre-test factors
Endoscopic specimens and those obtained by image-guided techniques should be fixed immediately in 10 % buffered formaldehyde, for <24 h. Resection specimens should be sent fresh to the pathology department as soon as possible. It is advisable to obtain macroscopic photographs for clinical/radiological/pathological correlation of staging, and to take frozen samples of tumour and normal tissue for possible subsequent use. Fixing in 10 % buffered formaldehyde should be carried out for 24–48 h. It is advisable to section the specimen to ensure that the whole tumour is properly fixed. Fragments fixed in the best conditions and adequately representative of the tumour should be chosen, avoiding any that were frozen during intra-operative assessment. Sections should not be stored at room temperature for longer than 14 days, and it is advisable to cover the section with layers of paraffin [30–33].
Test factors
The use of antigen retrieval systems and pre-validated antibodies is recommended. For Ki-67, the most suitable antibody is MIB-1.
Internal and external controls
It is essential to use positive and negative internal controls in each staining run. It is also important to assess intrinsic controls in the accompanying normal tissue. It is advisable for laboratories in charge of conducting biomarker tests to take part in quality control programmes, such as the one set up by the SEAP.
Pathology report
According to the WHO [17], the minimal data that should be contained in a pathology report on NETs of the digestive tract and pancreas must include the following items:
Gross examination
Specimens should be described according to the established protocols for each organ, including:
-
Specific site of origin of the lesion, i.e. oesophagus, stomach, duodenum, ampulla, jejunum, ileum, caecum, ascending, transverse, and descending colon, sigmoid colon, rectum, anus or pancreas.
-
Tumour size, given as the largest diameter of the tumour.
-
Extent of invasion: for tumours of the digestive tract, the level of invasion should be stated in the same terms used for exocrine tumours (lamina propria, muscularis mucosae, submucosa, muscularis propria, subserosa, serosa or adjacent tissues).
-
Distance from the tumour to the surgical margin, stating whether margins are negative, positive or very close.
Remarks Other guidelines consider it necessary to include the following findings:
-
Number of tumour lesions and number, site and size of regional lymph nodes [14, 34, 35]. Mark the most critical surgical margins with ink [34], take largest measurements of the tumour in three dimensions [6], note the presence of non-ischaemic tumour necrosis [14, 35] and record the degree of mesoappendiceal involvement (limited vs. extensive) [14].
-
In pancreatic tumours, it should be stated whether there is extrapancreatic spread or invasion of the bile duct, duodenum or ampulla [14].
-
In endoscopic biopsies, clinical information should include the site and size of lesions [35].
-
Other optional findings: distance of the tumour to the surgical margin, stated in millimetres, when <0.5 cm [14].
Microscopic examination
This examination should include the number of mitoses and the Ki-67 index. The number of mitoses is expressed per 10 HPFs, usually corresponding to an area of 2 mm2, and the Ki-67 index is given as a percentage, after examining 2,000 cells. These measurements should be obtained in several places in the most active areas.
Remarks
-
It is advisable to perform the mitotic count in 50 HPFs, even though it is subsequently expressed per 10 HPFs, as some guidelines recommend counting this amount of fields [14, 35].
-
To calculate the Ki-67 proliferative index, 2,000 tumour cells should be counted [34, 35]. When this is not feasible, e.g. in small biopsies, an “eyeballed” estimate is acceptable [14]. If the mitotic rate and Ki-67 proliferative index are inconsistent, the highest grade should be chosen [35]. It is worth noting, however, that in some series tumours with mitoses in the G2 range (<20/10 HPF) but a Ki-67 index in the G3 range (>20 %) have a better prognosis than G3 NECs with poorly differentiated NEC morphology or mitotic rate and Ki-67 index consistently within the grade 3 range. In needle biopsies, the Ki-67 index should be interpreted with caution. A recent study found that a certain percentage of G2 tumours might have been misinterpreted as G1, simply because tumour heterogeneity meant that the biopsy did not reflect the areas of greatest proliferation [36].
Other guidelines consider it necessary to include the following findings:
-
The existence of vascular and/or perineural invasion [14, 34, 35], the ratio of involved lymph nodes to total lymph nodes [14, 34, 35], the status of surgical margins [14, 34, 35] and local spread of the lesion, specifying whether there is invasion of the capsule and wall of the digestive tract [35].
-
The use of endothelial markers to confirm the presence of vascular invasion is optional and only recommended in cases of high histological suspicion [14].
-
There is disagreement about the use of general immunohistochemical markers, such as synaptophysin and chromogranin A [14, 34, 35] and about the inclusion in reports of architectural patterns, the presence of oncocytic cells, clear cells, gland formation, degenerative nuclear atypia and abnormalities or proliferative changes in tissue adjacent to the tumour (i.e. neuroendocrine hyperplasia in type 1 or 2 gastric NETs, pancreatic microadenomas in MEN-1, etc.) [34, 35]. Guidelines that regard the use of general neuroendocrine immunohistochemical markers as optional consider it necessary when examining poorly differentiated tumours and metastatic lesions [14].
-
For biopsies of metastatic tumours of unknown origin, in addition to the generic neuroendocrine markers, the immunohistochemical test panel should include tissue markers that point to a specific site, such as TTF-1 for lung, CDX-2 for intestine or pancreas and ISI-1 for pancreas. When interpreting these results, it must be remarked that negative findings with these markers do not rule out the site under investigation [14] and that TTF-1 may be positive in extrapulmonary NETs.
-
When assessing immunohistochemical stains, issues to bear in mind are the non-specificity of synaptophysin (positive in lesions of the adrenal cortex) and the peculiarities of chromogranin A staining (negative in rectal NETs and little or none in NECs) [34]. The diagnosis of NETs should be based on at least demonstrating one of the two neuroendocrine markers (synaptophysin and chromogranin A). If any of these are negative, the use of less specific markers, such as NSE, CD56, PGP9.5, CD57 and chromogranin B, is optional and may be considered [35]. Likewise, it is not essential to use CKs or p53, despite the fact that CK19 expression in pancreatic tumours has been said to entail an adverse prognosis [14].
-
Specific hormone markers for certain endocrine cell types found in the digestive tract and pancreas are optional and only recommended for use in certain circumstances, such as confirming the existence of a specific hormonal syndrome, identifying the cell lineage of gangliocytic paragangliomas, or in lymph-node or liver metastases of well-differentiated NETs of unknown primary. In the latter case, clues can be obtained about the possible primary origin of the lesion, e.g. the presence of serotonin points to an origin in the terminal ileum, the presence of gastrin suggests the duodenum or pancreas and the presence of glucagon points to the pancreas [34].
-
Also considered optional are immunostaining for somatostatin receptors, such as SSTR2, because this is useful for diagnosis and therapy [34], immunostaining with vascular markers to identify angio-invasion [14, 34], and the use of immunohistochemical markers potentially helpful for therapy (MGMT, VEGFR and mTOR) or for assessing biological aggressiveness (EGFR, HGF, CD44), as these have not been validated to justify their routine use [14].
-
If metastases have been resected, the site, number and dimensions of the largest lesion should be stated, as well as the percentage of organ involved [14].
Diagnosis
In order to make the pathological diagnosis, the following procedures must be carried out:
-
Classification of the lesions. The latest WHO classification (2010) [17] establishes two main categories, i.e. NET and NEC. Nevertheless, because of the recent change in terminology, the former terminology or another more classical terminology can optionally be added in brackets with the corresponding reference.
-
Tumour grade. This is based on the ENETS criteria, i.e. mitotic index and/or Ki-67 proliferative index.
-
TNM stage. Stages vary depending on the tumour site. The system used should be specified (ENETS and/or AJCC) as there are certain important differences between the two main staging systems (Table 1).
-
Type of cellularity and functionality. This should only be stated for clinical/pathological correlation. The suffix -OMA preceded by the hormone name should only apply to tumours with a clinical syndrome related to hormone overproduction. If the only evidence of hormone secretion comes from immunostaining, the wording used should be “NET with immunohistochemical demonstration of (specific hormone)”.
-
If the tumour has a mixed morphological phenotype with neuroendocrine and glandular epithelial structures, the extent of these should be specified. If one of them exceeds 30 % of the entire lesion, the term mixed adenoneuroendocrine carcinoma (MANEC) should be used [17, 35]. Combinations with squamous cell carcinoma or adenomas are very uncommon [35].
Remarks
-
No NET should be reported as benign, other than that in exceptional circumstances such as the subset of pancreatic or gastric tumours measuring <0.5 mm [14].
-
The main differences between the staging systems proposed by ENETS and the AJCC/Union for International Cancer Control (UICC) concern two main issues, i.e. the type of lesions included (the AJCC/UICC classification does not consider NECs) and the T-defining criteria at some specific sites, such as the pancreas and the appendix (Table 1) [16].
Clinical issues
Diagnostic serum biomarkers
General biomarkers
Chromogranin A is currently the most widespread serum biomarker for the diagnosis of GEP-NETs (Table 5). This is because it is co-secreted with other hormones by neuroendocrine cells, and this property persists when these cells become neoplastic. Chromogranin A has a diagnostic sensitivity of 60 % when all grades and stages of GEP-NETs are taken into consideration.
Various methods of testing for chromogranin A exist, the most sensitive and specific being radioimmunoassay (RIA) and ELISA [37]. However, proton pump inhibitor treatment, atrophic gastritis, inflammatory bowel disease, stress, essential arterial hypertension and kidney, heart or liver failure can raise Chromogranin A levels (false positives). Elevated chromogranin A can also occur in other malignant tumours of non-neuroendocrine lineage [37].
Chromogranin A levels tend to be correlated with tumour volume and the clinical course of the disease, and these markers are also useful for monitoring response to various treatment strategies [38]. NSE is another non-specific biomarker that can be raised in neuroendocrine tumours with a high tumour burden, poor histological differentiation or a high rate of cell death [38].
Specific biomarkers
For pancreatic NETs, pancreatic polypeptide may be useful for the early detection of NETs of pancreatic origin in the context of MEN-1, although this is controversial because of its low sensitivity (75 %). In the absence of a clinical syndrome to warrant it, serum tests for an extensive set of hormones are not indicated in non-functioning NETs. In contrast, functioning pancreatic tumours should be tested for specific biomarkers such as VIP, glucagon, somatostatin, GHRH or ACTH, as indicated by the patient’s clinical features [9, 39, 40].
With regard to intestinal NETs, endocrine tumours of the jejunum and ileum produce serotonin, but because of the hepatic first-pass effect this secretion only gives rise to carcinoid syndrome in patients who have developed liver metastases. Quantification of the urinary excretion of the principal metabolite of serotonin, 5-hydroxyindoleacetic acid (5-HIAA), is 73 % sensitive and 100 % specific for detecting the presence of an advanced NET of midgut origin [40, 41]. Duodenal tumours should be tested selectively for serum gastrin, somatostatin, 24-hour urinary 5-HIAA, GHRH and urinary cortisol, depending on the patient’s symptoms. Patients with MEN-1 should be tested for somatostatin, gastrin, chromogranin A, prolactin, glucagon, insulin, parathyroid hormone, plasma glucose and ionic calcium [42].
For tumours of colorectal origin, chromogranin A may be raised and could reflect tumour burden [40]. However, 5-HIAA is generally negative. Acid phosphatase levels may be elevated in tumours that test positive for prostate-specific acid phosphatase [43]. Levels of chorionic β-gonadotrophin may also be elevated [44].
A diagnosis of insulinoma can be established using six criteria [45], which include blood glucose levels (<40 mg/dl), insulin levels (≥6 μU/l), C-peptide levels (≥200 pmol/l), proinsulin levels (≥5 pmol/l), β-hydroxybutyrate levels (≤2.7 mmol/l) and absence of sulfonylurea in plasma and urine.
The diagnosis of gastrinoma is based on the existence of hypergastrinaemia (>100 pg/ml) in the presence of fasting hypersecretion of gastric acid. Whenever possible, proton pump inhibitors should be discontinued for at least a week and H2-blockers for 3 days before this test is performed. A gastrin value of over 1,000 pg/ml is highly suggestive of gastrinoma. However, 0.5 % of patients have normal serum gastrin levels. If fasting gastrin is elevated in the tenfold range and gastric pH is <2, a basal acid output (BAO) test should be done. Eighty-five per cent of patients with no history of gastric surgery will have a BAO of over 15 mEq/h [46]. Provocation tests, such as the secretin test, should be reserved for dubious cases, e.g. in patients with insufficiently high gastrin and acid hypersecretion.
Prognostic biomarkers
Chromogranin A
Chromogranin A is often elevated in GEP-NETs, with a sensitivity of 64–100 % and a specificity of 85 %, and the proportion of patients with elevated chromogranin A is higher the greater the extent of the disease. Thus, 73 % of patients with metastatic involvement compared with 26 % of those with localised disease have raised serum chromogranin A (p < 0.01), although this varies depending on the NET phenotype [47, 48]. Higher levels of chromogranin A have been described in gastrinomas, NETs of ileal origin and GEP-NETs associated with MEN-1.
A chromogranin A concentration three times above the upper limit of normal is associated with shorter survival time in patients with GEP-NETs, but chromogranin A levels also vary according to the grade of tumour differentiation. In a study of 63 patients with NETs, the diagnostic accuracy of chromogranin A was 76 % for well-differentiated NETs, 68 % for well-differentiated neuroendocrine carcinomas and 50 % for poorly differentiated tumours [47–50].
Chromogranin A is also a useful marker for assessing the therapeutic response, provided it is detectable before the intervention. Reductions in its serum concentration have been observed after surgery, liver transplantation, radionuclide therapy and various medical treatments [38, 47–52].
5-HIAA
Measuring 5-HIAA in 24-h collected urine is the most useful test for the diagnosis of carcinoid syndrome. It has a sensitivity of >70 % and a specificity of close to 100 % if false positive elevations caused by the intake of some foods or drugs are ruled out. In carcinoid tumours of the midgut, urinary 5-HIAA levels are related to tumour volume and disease prognosis. Carcinoid tumours of the foregut, mainly bronchial carcinoids, tend not to produce 5-hydroxytryptophan (5-HTP). However, they sometimes produce an atypical carcinoid syndrome as a result of histamine secretion by the tumour. In these cases, testing for histamine and its metabolites in 24-h urine may be useful for diagnosis and monitoring of the disease [53].
NSE
NSE is located in the cytoplasm and, unlike chromogranin A, is not secreted. Its diagnostic sensitivity in GEP-NETs is low (32–47 %) and similar in carcinoid and pancreatic tumours (42–47 % and 37–45 %, respectively). Its plasma concentrations tend to rise in more aggressive tumours (G3, high tumour burden) and this is associated with a worse prognosis [49–52].
Ki-67
Various studies confirm the prognostic value of the Ki-67 index, with a higher proliferative index associated with a lower survival. In fact, the most recent NET classification systems (ENETS or the WHO classification) include it as a fundamental part of the assessment and prognostic stratification of these tumours [7, 54, 55]. Evidence also suggests that the higher the proliferative index, the more chance there is that a tumour will respond to conventional cytotoxic chemotherapy treatment. Thus, no benefit is likely to be seen with tumours that have proliferative indices of <10 %.
Predictive biomarkers
The search for biomarkers predictive of response or resistance to cancer treatments has experienced a boom in the past few years with the advent of new targeted therapies. The aim of these predictive biomarkers is to improve selection of patients most likely to benefit from these agents and/or to avoid costly, toxic treatments in refractory patients. This allows to improve the risk/benefit ratio of these therapies, and to optimise the rational use of available resources.
Angiogenesis inhibitors
In general, NETs are well-vascularised tumours with high expression of molecular markers related to angiogenesis and involved in tumour proliferation and growth [56]. In a Phase II study evaluating the efficacy of sunitinib treatment in 109 patients with metastatic NETs, plasma levels of various soluble proteins related to the angiogenesis process were analysed: VEGF, sVEGFR2, sVEGFR3 and IL-8. The greatest clinical benefit was seen in patients with high baseline levels of sVEGFR2 and VEGF and low baseline levels of IL-8, and in patients with greater reductions in sVEGFR2-3 levels and greater increases in IL-8 levels during treatment [57].
Unfortunately, the randomised Phase III study that granted sunitinib approval for the treatment of pancreatic NETs did not analyse these biomarkers, so the chance to develop valuable tools to enable better patient selection in clinical practice was lost. In a recent observational study, polymorphisms in CYP3A5, VEGF-A and VEGFR2 were determined in 24 patients with NETs treated with sunitinib and correlated with the likelihood of developing treatment-induced toxicity. The results showed that polymorphisms rs699947 in VEGF-A and rs776746 in CYP3A5 were predictive factors for greater toxicity and the risk of dose reduction [58].
mTOR inhibitors
The PI3K-AKT-mTOR pathway plays a major role in the pathogenesis of NETs. Protein expression and molecular profiling studies have demonstrated loss of expression of tuberous sclerosis protein 2 (TSC2) and phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase (PTEN) in 80 % of pancreatic NETs, as well as mutations in various genes in the mTOR pathway in 15 % of cases [27, 59]. Although loss of TSC2 and PTEN gene expression has been linked to patient prognosis, the correlation between the various molecular alterations described and response to inhibitors of the mTOR pathway has not yet been evaluated in clinical practice.
However, a recently published study analysed mTOR pathway status in various rapamycin-sensitive and rapamycin-resistant cell lines in murine models of NETs and breast cancer and in paired biopsies from 19 patients with NETs treated with everolimus and octreotide. The study demonstrated that rapamycin-sensitive cell lines more often harboured mutations in PI3KCA and PTEN and had greater AKT phosphorylation. Also, patients with high baseline levels of phosphorylated AKT and a greater increase in phosphorylated AKT during treatment had longer progression-free survival and were more likely to achieve a partial response to treatment [60]. The value of molecular alterations in PI3KCA/PTEN, as well as baseline levels and changes in phosphorylated AKT, in predicting the response to mTOR inhibitors should be validated in future prospective trials properly designed for that purpose.
MGMT
O6-methylguanine-DNA methyltransferase (MGMT) is an enzyme that repairs DNA damage induced by alkylating agents. The absence or low expression of tumour MGMT as measured by immunohistochemical techniques has been associated with greater sensitivity to these drugs in the context of various cancers [61]. In the NET setting, loss of expression of this enzyme has been observed in approximately half of tumours of pancreatic origin, but is extremely rare in tumours of intestinal origin. Retrospective studies suggest that MGMT deficiency is associated with greater sensitivity to temozolomide treatment [62].
Functional and molecular imaging
Octreotide scan
Octreotide scanning is the standard tool for diagnosis, localisation, staging and selection of patients eligible for treatment with somatostatin analogues or radionuclides [63]. This technique employs the radioactive tracer 111In-D-Phe-octreotide, which is highly sensitive for detecting tissue with somatostatin receptors and has a long half-life, which requires readings to be made 24 and 48 h after its administration. In patients being treated with somatostatin analogues, the octreotide scan should be done just before the next dose is given, to prevent false negatives. On the other hand, this technique can give rise to false positives in patients with chronic inflammatory diseases, granulomatosis, haemangiomas, recent surgery, or other malignancies such as cholangiocarcinoma, lymphoma, breast cancer or prostate cancer.
Expression of somatostatin receptors, and hence sensitivity to this test, is 80 % in enteric carcinoid tumours and 50–80 % in NETs of pancreatic origin. Within these two large groups, however, sensitivity varies depending on the subtype of NET, being over 90 % in NETs of midgut origin, over 80 % in gastrinomas, 70 % in PPomas, glucagonomas and VIPomas, but less than 50 % in insulinomas. The specificity of this technique, however, is close to 100 %, being higher with a combination of octreotide scan, SPECT and CT scan [64].
MIBG
Scintigraphy with 123I-metaiodobenzylguanidine (MIBG), a noradrenaline analogue, is the investigation of choice for phaeochromocytomas or paragangliomas, but is less sensitive in carcinoid tumours, in which it is used when other tests fail or to decide on the potential indication of MIBG treatment [65].
PET (HTP, DOPA, 68Ga, FDG)
The existence of a very active amino-acid transporter and of cell-membrane hormone receptors in NET cells explains the high affinity of these tumours for radiopharmaceuticals such as 18F-DOPA and, especially, gallium-68 DOTA (68Ga-DOTA) [66] and HTP, which are used in positron emission tomography (PET) and allow the detection of up to 20–30 % more lesions than with other tests [67]. The advantages over octreotide scanning include greater spatial resolution (of up to 3 mm), sensitivity and specificity. They also have greater ability to detect tumours expressing low levels of somatostatin receptors, such as insulinomas, which can be negative in octreotide scans. Moreover, this technique is more convenient for the patient as additional visits 24 and 48 h after administration of the radiopharmaceutical are not required [68]. Poorly differentiated NECs, with no hormone secretion, tend not to be detected by either of these techniques (octreotide scan or PET with HTP, 18F-DOPA or 68Ga-DOTA). However, these tumours, which generally have a high proliferative index (Ki-67), tend to show high activity with radiopharmaceuticals such as 18F-fluorodeoxyglucose (FDG), which is the one most commonly used in neoplasms of non-neuroendocrine lineage [69, 70].
Evaluation of familial cancer risk
GEP-NETs tend to be sporadic, but can occasionally appear in the context of familial cancer syndromes such as MEN-1 or VHL. A discussion of the criteria and applications for diagnostic tests in these contexts is beyond the scope of these guidelines. However, in the presence of clinical evidence (young patients, family history, or coexistence of other tumours typical of these syndromes) or suggestive pathology (multiple tumours, islet cell dysplasia, clear cells) it is advisable for molecular tests to begin at the level of the index cases.
Conclusions
This article includes a discussion of the main clinical and pathological features of GEP-NETs, together with recommendations for clinicians and pathologists on how to use current available serum and tissue markers in this context. It should be stressed that this document reflects the majority views agreed at the time of writing.
References
Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72.
Garcia-Carbonero R, Salazar R, Sevilla I, et al. SEOM clinical guidelines for the diagnosis and treatment of gastroenteropancreatic neuroendocrine tumours (GEP NETS). Clin Transl Oncol. 2011;13:545–51.
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Lawrence B, Gustafsson BI, Chan A et al (2011) The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 40:1–18, vii.
Benavent M, de Miguel MJ, Garcia-Carbonero R. New targeted agents in gastroenteropancreatic neuroendocrine tumors. Target Oncol. 2012;7:99–106.
Ploeckinger U, Kloeppel G, Wiedenmann B, et al. The German NET-registry: an audit on the diagnosis and therapy of neuroendocrine tumors. Neuroendocrinology. 2009;90:349–63.
Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE). Ann Oncol. 2010;21:1794–803.
Williams ED, Sandler M. The classification of carcinoid tumours. Lancet. 1963;1:238–9.
Oberndorfer S. Karzinoide Tumoren des Dünndarms. Frankfurter Zeitschrift för Pathologie. 1907;1:425–32.
Capella C, Heitz PU, Hofler H, et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425:547–60.
Capella C, Riva C, Rindi G, et al. Histopathology, hormone products, and clinicopathological profile of endocrine tumors of the upper small intestine: a study of 44 cases. Endocrine Pathology. 1991;2:92–110.
Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18(Suppl 1):S1–16.
Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84.
Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–13.
Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12.
Kloppel G, Rindi G, Perren A, et al. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Arch. 2010;456:595–7.
Bosman F, Carneiro F, Hruban R, et al. WHO classification of tumours of the disgestive system. France: Lyon; 2010.
Adsay V. Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: to count or not to count is not the question, but rather how to count. Am J Surg Pathol. 2012;36:1743–6.
Tang LH, Gonen M, Hedvat C, et al. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol. 2012;36:1761–70.
Annenkov A, Nishikura K, Domori K, et al. Alpha-methylacyl-coenzyme A racemase expression in neuroendocrine neoplasms of the stomach. Virchows Arch. 2012;461:169–75.
Aizawa M, Kojima M, Gotohda N, et al. Geminin expression in pancreatic neuroendocrine tumors: possible new marker of malignancy. Pancreas. 2012;41:512–7.
La Rosa S, Marando A, Furlan D, et al. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. 2012;36:601–11.
Chan ES, Alexander J, Swanson PE, et al. PDX-1, CDX-2, TTF-1, and CK7: a reliable immunohistochemical panel for pancreatic neuroendocrine neoplasms. Am J Surg Pathol. 2012;36:737–43.
Panarelli NC, Yantiss RK, Yeh MM, et al. Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2. Am J Clin Pathol. 2012;138:211–22.
Verbeke CS. Endocrine tumours of the pancreas. Histopathology. 2010;56:669–82.
Fernandes I, Pacheco TR, Costa A, et al. Prognostic significance of AKT/mTOR signaling in advanced neuroendocrine tumors treated with somatostatin analogs. Onco Targets Ther. 2012;5:409–16.
Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245–55.
Wang H, Chen Y, Fernandez-Del Castillo C, et al. Heterogeneity in signaling pathways of gastroenteropancreatic neuroendocrine tumors: a critical look at notch signaling pathway. Mod Pathol. 2013;26:139–47.
Lorenzo PI, Jimenez Moreno CM, Delgado I, et al. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochem Cell Biol. 2011;136:595–607.
Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2011;135:537–43.
Bai Y, Tolles J, Cheng H, et al. Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Lab Invest. 2011;91:1253–61.
Munakata S, Hendricks JB. Effect of fixation time and microwave oven heating time on retrieval of the Ki-67 antigen from paraffin-embedded tissue. J Histochem Cytochem. 1993;41:1241–6.
Wester K, Wahlund E, Sundstrom C, et al. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8:61–70.
Kloppel G, Couvelard A, Perren A, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–6.
Rindi G, Bordi C, La Rosa S, et al. Gastroenteropancreatic (neuro)endocrine neoplasms: the histology report. Dig Liver Dis. 2011;43(Suppl 4):S356–60.
Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–60.
Molina R, Alvarez E, Aniel-Quiroga A, et al. Evaluation of chromogranin A determined by three different procedures in patients with benign diseases, neuroendocrine tumors and other malignancies. Tumour Biol. 2011;32:13–22.
Oberg K. Circulating biomarkers in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2011;18(Suppl 1):S17–25.
O’Toole D, Salazar R, Falconi M, et al. Rare functioning pancreatic endocrine tumors. Neuroendocrinology. 2006;84:189–95.
Ardill JE, Erikkson B. The importance of the measurement of circulating markers in patients with neuroendocrine tumours of the pancreas and gut. Endocr Relat Cancer. 2003;10:459–62.
Feldman JM, O’Dorisio TM. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. Am J Med. 1986;81:41–8.
Witzigmann H, Loracher C, Geissler F, et al. Neuroendocrine tumours of the duodenum. Clinical aspects, pathomorphology and therapy. Langenbecks Arch Surg. 2002;386:525–33.
Davidson ED, McDougal WS. Elevated serum acid phosphatase levels with rectal carcinoid tumor. Gastroenterology. 1976;70:114–6.
Norheim I, Oberg K, Theodorsson-Norheim E, et al. Malignant carcinoid tumors. An analysis of 103 patients with regard to tumor localization, hormone production, and survival. Ann Surg. 1987;206:115–25.
Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144–52.
Roy PK, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome. Correlation with clinical expression, tumor extent and role in diagnosis—a prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine (Baltimore). 2001;80:189–222.
Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967–73.
Lawrence B, Gustafsson BI, Kidd M et al (2011) The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 40:111–134, viii.
Yao JC, Pavel M, Phan AT, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–9.
Nobels FR, Kwekkeboom DJ, Coopmans W, et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622–8.
O’Toole D, Couvelard A, Rebours V, et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:847–56.
Gilbert JA, Adhikari LJ, Lloyd RV, et al. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr Relat Cancer. 2010;17:623–36.
O’Toole D, Grossman A, Gross D, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: biochemical markers. Neuroendocrinology. 2009;90:194–202.
Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33.
Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–803.
Fjallskog ML, Lejonklou MH, Oberg KE, et al. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res. 2003;9:1469–73.
Bello C, Deprimo SE, Friece C, et al. (2006) Analysis of circulating biomarkers of sunitinib malate in patients with unresectable neuroendocrine tumors (NET): VEGF, IL-8, and soluble VEGF receptors 2 and 3. ASCO Meeting Abstracts 24:Abstract 4045.
Rodriguez de Antona C, Grande Pulido E, Leandro-Garcia L, et al. (2012) Evaluation of CYP3A5, VEGF-a, and VEGFR2 polymorphisms as markers of sunitinib toxicity. ASCO Meeting Abstracts 30:Abstract 10546.
Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203.
Meric-Bernstam F, Akcakanat A, Chen H, et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin Cancer Res. 2012;18:1777–89.
Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–4.
Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338–45.
Alexandraki KI, Kaltsas G. Gastroenteropancreatic neuroendocrine tumors: new insights in the diagnosis and therapy. Endocrine. 2012;41:40–52.
Oberg K, Eriksson B. Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:265–76.
Bombardieri E, Giammarile F, Aktolun C, et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37:2436–46.
Ambrosini V, Tomassetti P, Castellucci P, et al. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:1431–8.
Naji M, Hodolic M, El-Refai S, et al. Endocrine tumors: the evolving role of positron emission tomography in diagnosis and management. J Endocrinol Invest. 2010;33:54–60.
Wong KK, Arabi M, Zerizer I, et al. Role of positron emission tomography/computed tomography in adrenal and neuroendocrine tumors: fluorodeoxyglucose and nonfluorodeoxyglucose tracers. Nucl Med Commun. 2011;32:764–81.
Abgral R, Leboulleux S, Deandreis D, et al. Performance of (18)fluorodeoxyglucose-positron emission tomography and somatostatin receptor scintigraphy for high Ki67 (≥10%) well-differentiated endocrine carcinoma staging. J Clin Endocrinol Metab. 2011;96:665–71.
Ambrosini V, Tomassetti P, Rubello D, et al. Role of 18F-dopa PET/CT imaging in the management of patients with 111In-pentetreotide negative GEP tumours. Nucl Med Commun. 2007;28:473–7.
Acknowledgments
The members of the Working Group on Biomarkers SEOM-SEAP are Ramón Colomer, Pilar García-Alfonso, Pilar Garrido, Ricardo González Cámpora, José Palacios and Enrique de Álava.
Conflict of interest
SEOM and SEAP acknowledge the financial support for this project of unrestricted grants from Pfizer Oncology and Novartis Oncology. Jorge Barriuso has been partially funded by The Spanish Association Against Cancer. The authors state that, at the time of drafting and revision the text of the manuscript, they were unaware of the names of the laboratories who have supported this project, so this support has not influenced the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Carbonero, R., Vilardell, F., Jiménez-Fonseca, P. et al. Guidelines for biomarker testing in gastroenteropancreatic neuroendocrine neoplasms: a national consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol 16, 243–256 (2014). https://doi.org/10.1007/s12094-013-1062-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1062-9