Abstract
Background and aims
Because the outcome of glioblastoma multiforme (GBM) remains dismal, there is an urgent need for a better molecular characterization of this malignancy. The aim of this prospective study was to investigate the prognostic impact of the expression of c-mesenchymal-epithelial transition (c-Met) a receptor tyrosine kinase implicated in expression growth, survival, motility/migration, and invasion in GMB patients managed according to the established diagnostic and therapeutic protocols.
Methods
Between May 2003 and March 2011, a total of 69 patients (33 males and 36 females; mean age: 52.2 ± 12.9 years, age range: 23–81 years) referred to our Department for the surgical removal of GBM were evaluated immunohistochemically for c-Met expression. Progression-free survival (PFS) and overall survival (OS) served as the main outcome measures.
Results
Compared with c-Met− subjects (n = 38), c-Met+ subjects (n = 31) had both a significantly lower OS (15.3 ± 2.3 vs. 22.6 ± 2.5 months, respectively, p < 0.01) and PFS (12.3 ± 2.1 vs. 19.1 ± 2.6 months, respectively, p < 0.05). After allowance for potential confounders, multivariate Cox regression analysis identified c-Met+ as an independent predictor of both OS (hazard ratio = 1.7; 95 % confidence interval = 1.2–1.9, p < 0.01) and PFS (hazard ratio = 1.6; 95 % confidence interval = 1.1–2.3, p < 0.05).
Conclusions
Our findings suggest that c-Met immunohistochemical expression is an independent predictor of outcomes in patients with GBM treated by standard of care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most malignant of the astrocytic gliomas and constitutes the most common malignant primary brain tumor [1]. Despite being a relatively rare malignancy with a global incidence rate of only 3.17 cases per 100,000 [2], GBM is an aggressive disease characterized by a poor prognosis with a median survival time of only 12–15 months from the time of diagnosis [3]. The main reasons for the poor prognosis of GBM are the late stage of diagnosis combined with lack of efficacy of currently available therapies (maximal exeresis, combined radio- and chemotherapy, and adjuvant chemotherapy) [4]. Because the outcome of this tumor remains dismal and its optimal management is still debated, there is an urgent the need for a better molecular characterization of GBM which would ultimately improve both classification and treatment [5]. Accordingly, the complex biology of GBM is reflected by a marked biological heterogeneity. Consequently, there has been great interest in identifying prognostic factors influencing tumor recurrence and survival [6].
By regulating cell functions such as growth, survival, motility/migration, and invasion, the c-mesenchymal-epithelial transition (c-Met) receptor tyrosine kinase has been implicated in the pathogenesis and prognosis of many human malignancies [7, 8]. The human c-Met gene has been as been localized to 7q21–q31 and encodes a 1368 aminoacid protein organized in a short α- and a long β-chain. The α-chain and the first portion of the β-chain are essential for the receptor ligand interaction. Moreover, the β-chain contains the ectocellular domain, as well as the transmembrane domain and the intracellular kinase domain [7, 8]. Deregulated c-Met activation, caused by gene amplification, translocation, mutation or autocrine/paracrine hepatocyte growth factor (HGF) signaling, has been reported in different solid tumors such as B cell lymphoma, bladder cancer, breast cancer, colorectal cancer, and ovarian cancer [9, 10]. Growing evidence also points to a key role of c-Met in the pathogenesis of GBM. Shiota et al. [11] reported that the coexpression of HGF and c-Met stimulates the growth of HGL4 glioblastoma cells. Uchinokura and co-workers [12] demonstrated the existence of multiple pathways of c-Met activation in glioblastomas. From a clinical standpoint, Liu et al. [13] have reported that the expression of c-Met differs significantly between primary and recurrent GBMs, and patients with tumors expressing c-Met at a higher level had a significantly shorter progression-free survival (PFS) time. The prognostic significance of c-Met expression in tumor specimens is assuming increasing importance given the therapeutic advances in the use of c-Met inhibitors in several types of cancer, including GBM. Notably, Xie et al. [14] have recently suggested that c-Met activity may serve as a clinically useful predictive marker that can identify specific subgroups of GBM patients who may be candidates for targeted treatment with c-Met inhibitors. Accordingly, c-Met has been repeatedly proposed as a potential therapeutic target in GBM [15–17]. In this prospective study, we examined whether c-Met expression evaluated immunohistochemically can be related to the prognosis of Turkish patients with GBM managed according to the established diagnostic and therapeutic protocols.
Materials and methods
Patients
Between May 2003 and March 2011, a total of 69 patients (33 males and 36 females; mean age: 52.2 ± 12.9 years, age range: 23–81 years) referred to the Department of Oncology, Uludag University Medical School (Bursa, Turkey) for surgical removal of GBM were enrolled in the study. All of the patients were managed according to the established diagnostic and therapeutic protocols [4], including surgical resection and subsequent chemoradiotherapy. Patient data included sex, age, type of GBM (primary vs. secondary), radiographic pattern, type of surgical resection, Karnofsky performance status, use of chemotherapy, type of second-line treatment, and the proliferative cell marker Ki-67. The study was performed according to the Declaration of Helsinki, and approval was granted by the Institutional Review Board of the Uludag University School of Medicine. All participants provided written informed consent.
Histopathology and c-Met expression
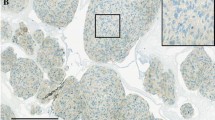
Tumor samples collected during surgery or biopsy were embedded in paraffin and evaluated by experienced neuropathologists. The Ki-67 labeling index was expressed as the number of positive cells per 1,000 cells [18]. One appropriate block from each specimen was selected and serial 4-μm-thick sections of tissue measuring at least 0.5 × 0.5 cm were cut from the paraffin lock. The sections were used for immunohistochemical analysis of c-Met expression. Sections were deparaffinized in turpentine for 30 min and rehydrated in a graded ethanol series (100, 95, 90, 85, and 75 %) for 5 min each. Heat-induced antigen retrieval (10 mM citrate buffer, pH 6.0) was performed for 15 min at 95 °C. Endogenous peroxidase activity was blocked by incubating sections in 0.3 % hydrogen peroxide in methanol for 30 min. After a washing step with phosphate-buffered saline (PBS, pH 7.2), sections were blocked with normal goat serum (Zhongshan Golden Bridge Biotechnology, Beijing, China) for 30 min at room temperature. The tissue sections were subsequently incubated overnight at 4 °C with a primary antibody against c-Met [Met Rabbit monoclonal antibody (EP1454Y), N-term, 1:100 dilution; GeneTex Inc., Alton Parkway Irvine, CA, USA]. Sections were then washed in PBS, and incubated with secondary antibodies (Zhongshan Golden Bridge Biotechnology, 1:100 dilution) for 1 h. Subsequent reactions were carried out using a streptavidin–biotin-peroxidase method. Diaminobenzidine tetrahydrochloride was used as the enzyme substrate to observe the specific antibody localization, and Harris hematoxylin was used as a nuclear counterstain. Human breast carcinoma tissue served as positive control. All of the specimens were evaluated by a single expert pathologist.
Scoring for c-Met expression
Sections were examined for immunoreactivity for c-Met using high-powered fields in areas with maximal staining. Tumors were divided into four categories, as follows [19]: grade 0, no expression; grade 1, fewer than 30 % of cells expressing c-Met; grade 2, between 30 and 60 % of cells expressing c-Met; grade 3, more than 60 % of cells expressing c-Met in the high-power field in areas with maximal staining. Tumors were scored positive (c-Met+) if more than 30 % of cells expressed c-Met (i.e. grades 2–3).
Statistical analysis
Descriptive statistics included counts, means, and standard errors, as appropriate. Overall survival (OS) was calculated from the date of surgery to the date of last follow-up or date of death. Progression-free survival was calculated beginning with the date of first surgery until recurrence or last follow-up. Survival curves were drawn using the Kaplan–Meier method, and the differences between the groups were compared with the log-rank test. Univariate and multivariate analyses using Cox regression through a forward selection procedure were used to identify the independent predictors of outcomes. The appropriateness of the proportional hazards assumption was verified using graphical methods and tested as described by Grambsch and Therneau [20]. The assumption of linearity for the Cox models was examined by visual inspection, and no violation was found. All of the variables listed in Table 1 were tested for their association with the study outcomes using univariate analysis. Only variables that were statistically significant in univariate analysis were entered into the multivariate Cox regression model. All calculations were performed using the SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 4.0 (GraphPad Inc., San Diego, CA, USA). A two-tailed p < 0.05 was considered statistically significant.
Results
General characteristics
The median follow-up time was 32 months (minimum–maximum: 3–95 months). The general characteristics of the study patients are shown in Table 1. Of a total of 69 patients with GBM, 54 (78 %) had solitary lesions whereas the tumors were invasive/multifocal in 15 patients (22 %). Fifty-four patients (78 %) underwent macroscopic total resection, ten a partial resection (14 %), and five (8 %) biopsy only. The majority of patients (n = 61, 88.4 %) had primary GBM. A total of 61 patients (88.4 %) received temozolomide. At the time of the last follow-up, 66 patients (96 %) were dead, whereas three (4 %) were alive.
c-Met expression
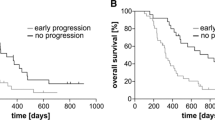
The c-Met expression score was 0 in 19 patients (27 %), 1 in 19 patients (27 %), 2 in 15 patients (21 %), and 3 in 16 patients (25 %). Therefore, a total of 31 patients (45 %) were classified as c-Met+. The c-Met expression score did not correlate with the general characteristics of the study participants. The mean OS was 19.2 ± 1.7 months, whereas the mean PFS was 16.1 ± 1.6 months. Compared with c-Met− subjects (n = 38), c-Met+ subjects (n = 31) had both a significantly lower OS (15.3 ± 2.3 vs. 22.6 ± 2.5 months, respectively, p < 0.01; Fig. 1) and PFS (12.3 ± 2.1 vs. 19.1 ± 2.6 months, respectively, p < 0.05; Fig. 2).
Predictors of clinical outcomes
We then tested the association between all of the variables listed in Table 1 with both OS and PFS. The results of univariate Cox regression analysis are shown in Table 2. Only age and c-Met+ were identified as associated with both OS and PFS in our sample. In multivariate Cox regression analysis, c-Met+ retained its independent prognostic significance for both OS (hazard ratio = 1.7; 95 % confidence interval = 1.2–1.9, p < 0.01) and PFS (hazard ratio = 1.6; 95 % confidence interval = 1.1–2.3, p < 0.05).
Discussion
The identification of novel prognostic markers may help to better assess survival probability in different subgroups of GBM patients and to tailor treatment according to the molecular profile of the tumor [1]. The results of this study indicate that an increased immunohistochemical expression of c-Met in GBM tumor samples is significantly and independently associated with poor OS and PFS. Conversely, the data obtained herein significantly demonstrated that the patients with diminished expression of c-Met had greater survival rates when compared with the group of patients with high immunohistochemical expression of this molecule, which endorses the previous results by Liu et al. [13] who showed that highly expressed c-Met in GBM is significantly related to a shorter PFS time in a series of 19 Chinese patients. Similar to Liu et al. [13], in this study we opted for immunohistochemical evaluation of c-Met because this method is reproducible and easily applicable in routine of pathology. Of note, it has been demonstrated that an increased c-Met immunohistochemical expression is significantly associated with high level of c-Met mRNA and gene amplification [21]. Our results confirm and expand those by Liu and coworkers [13] in a larger and ethnically diverse clinical population, and suggest that c-Met immunohistochemical expression may predict OS in GBM patients. These results are also in line with those obtained by Kong and colleagues [22], who showed that c-Met overexpression in a series of 62 Korean patients with GBM is associated with shorter survival time and poor treatment response, the mechanism for which could be related to a higher tumor invasiveness on the molecular and clinical phenotypes.
To date, several studies have shown that c-Met may serve as a potential therapeutic target in different solid tumors [23] and numerous anti-c-Met drugs are in preclinical and clinical testing as anticancer drugs [9, 10]. For example, the compound INCB28060 has been shown to exhibit picomolar enzymatic potency and is highly specific for c-Met [24]. The use of c-Met inhibitors is particularly appealing in GBM patients, because certain glioblastomas display HGF autocrine activation of the c-Met signaling pathway [14], ultimately resulting in tumor proliferation, cell survival, migration, invasion, and angiogenesis. In this regard, Guessous et al. [16] have reported that the oral delivery of a small molecule kinase inhibitor of c-Met inhibits intracranial tumor growth to mice bearing orthotopic human glioblastoma xenografts. It is also worth noting that the small-molecule c-Met inhibitor MP470 has been shown to radiosensitize glioblastoma cells in vitro and in vivo, thereby potentially improving the outcomes of patients with GBM [15].
In accordance with previous studies, [25–27], the results from this report confirm that age at diagnosis is clearly important as a prognostic factor in GBM patients. This finding is consistent with the results seen in meta-analyses indicating that inclusion of adjuvant chemotherapy for GBM patients provides an increase in survival, although that improvement tends to be minimal for patients over 65 years [28, 29].
The limitations of this study are the homogeneous ethnic background of the study population and that the patients included in the study could be a selected group of Turkish patients and may not represent the general population of patients with GBM. Another potential caveat inherent in this study is that all of the specimens were reviewed by a single pathologist. These limitations notwithstanding, our present findings have provided additional and updated information on the prognostic significance of c-Met overexpression in GBM patients. Some of the findings presented will prove immediately clinically useful for the prediction of prognosis and risk stratification. In most cases, the clinical behavior of GBM can be predicted with relative accuracy based on the combination of age and c-Met expression. Hopefully, these data can make some contribution towards improving the outlook for future patients with these rare tumors. Beyond clinical applicability, future work must address mechanistic questions about the functional role of c-Met expression in determining the outcome of GBM.
References
Rekers NH, Sminia P, Peters GJ. Towards tailored therapy of glioblastoma multiforme. J Chemother. 2011;23:187–99.
Gilbert CA, Daou MC, Moser RP, Ross AH. Gamma-secretase inhibitors enhance temozolomide treatment of human gliomas by inhibiting neurosphere repopulation and xenograft recurrence. Cancer Res. 2010;70:6870–9.
Tran B, Rosenthal MA. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010;17:417–21.
Zhang X, Zhang W, Cao WD, Cheng G, Zhang YQ. Glioblastoma multiforme: molecular characterization and current treatment strategy (review). Exp Ther Med. 2012;3:9–14.
Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, et al. Glioblastoma multiforme: a review of therapeutic targets. Exp Opin Ther Targ. 2009;13:701–18.
Masui K, Cloughesy TF, Mischel PS. Molecular pathology in adult high-grade gliomas from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol. 2012;38:271–91.
Jung KH, Park BH, Hong SS. Progress in cancer therapy targeting c-Met signaling pathway. Arch Pharm Res. 2012;35:595–604.
Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103.
Cañadas I, Rojo F, Arumí-Uría M, Rovira A, Albanell J, Arriola E. C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin Transl Oncol. 2010;12:253–60.
Yap TA, Sandhu SK, Alam SM, de Bono JS. HGF/c-MET targeted therapeutics: novel strategies for cancer medicine. Curr Drug Targ. 2011;12:2045–58.
Shiota G, Kawasaki H, Nakamura T. Coexpression of hepatocyte growth factor and its receptor (c-Met oncogene) in HGL4 glioblastoma cells. Oncology. 1996;53:511–6.
Uchinokura S, Miyata S, Fukushima T, Itoh H, Nakano S, Wakisaka S, et al. Role of hepatocyte growth factor activator (HGF activator) in invasive growth of human glioblastoma cells in vivo. Int J Cancer. 2006;118:583–92.
Liu W, Fu Y, Xu S, Ding F, Zhao G, Zhang K, et al. c-Met expression is associated with time to recurrence in patients with glioblastoma multiforme. J Clin Neurosci. 2011;18:119–21.
Xie Q, Bradley R, Kang L, Koeman J, Ascierto ML, Worschech A, et al. Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci USA. 2012;109:570–5.
Welsh JW, Mahadevan D, Ellsworth R, Cooke L, Bearss D, Stea B. The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat Oncol. 2009;4:69.
Guessous F, Zhang Y, diPierro C, Marcinkiewicz L, Sarkaria J, Schiff D, et al. An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth. Anticancer Agents Med Chem. 2010;10:28–35.
Buchanan SG, Hendle J, Lee PS, Smith CR, Bounaud PY, Jessen KA, et al. SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol Cancer Ther. 2009;8:3181–90.
Terzolo M, Boccuzzi A, Bovio S, Cappia S, De Giuli P, Alì A, et al. Immunohistochemical assessment of Ki-67 in the differential diagnosis of adrenocortical tumors. Urology. 2001;57:176–82.
Hou XZ, Liu W, Fan HT, Liu B, Pang B, Xin T, et al. Expression of hepatocyte growth factor and its receptor c-Met in human pituitary adenomas. Neurol Oncol. 2010;12:799–803.
Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, et al. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J. 2006;27:802–7.
Huang TJ, Wang JY, Lin SR, Lian ST, Hsieh JS. Overexpression of the c-met protooncogene in human gastric carcinoma—correlation to clinical features. Acta Oncol. 2001;40:638–43.
Kong DS, Song SY, Kim DH, Joo KM, Yoo JS, Koh JS, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115:140–8.
Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26.
Liu X, Wang Q, Yang G, Marando C, Koblish HK, Hall LM, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17:7127–38.
Barker FG 2nd, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB, et al. Age and radiation response in glioblastoma multiforme. Neurosurgery. 2001;49:1288–97.
Pierga JY, Hoang-Xuan K, Feuvret L, Simon JM, Cornu P, Baillet F, et al. Treatment of malignant gliomas in the elderly. J Neurooncol. 1999;43:187–93.
Whittle IR, Basu N, Grant R, Walker M, Gregor A. Management of patients aged >60 years with malignant glioma: good clinical status and radiotherapy determine outcome. Br J Neurosurg. 2002;16:343–7.
Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neurol Oncol. 2004;6:227–35.
Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8.
Conflict of interest
The authors declare that they have no conflict of interest relating to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olmez, O.F., Cubukcu, E., Evrensel, T. et al. The immunohistochemical expression of c-Met is an independent predictor of survival in patients with glioblastoma multiforme. Clin Transl Oncol 16, 173–177 (2014). https://doi.org/10.1007/s12094-013-1059-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-013-1059-4